
Movement Disorder Emergencies: Serotonin Syndrome and Neuroleptic Malignant Syndrome
August 1, 2025
By Monique Graf, MD; Amy Young, MD; Fernando Benitez, MD; and Larissa Velez, MD
Executive Summary
- Serotonin syndrome and neuroleptic malignant syndrome are both pharmacologically induced conditions with altered mental status, neuromuscular abnormalities, and autonomic dysfunction.
- Serotonin syndrome is caused by excessive stimulation of the postsynaptic serotonin receptor.
- Neuroleptic malignant syndrome is caused by disruption of dopamine signaling, resulting in a relative state of dopamine deficiency.
- The more severe cases of serotonin syndrome result from synergistic interaction of two or more serotonergic drugs used at the same time.
- Neuroleptic malignant syndrome can be caused by dopamine antagonists or abrupt discontinuation of dopaminergic drugs.
- The onset with serotonin syndrome typically is quicker, within 24 hours, than with neuroleptic malignant syndrome, which typically is days to weeks.
- The key neuromuscular distinction between the two syndromes is akathisia, tremor, hyperreflexia and clonus (more prominent in the lower extremities in serotonin syndrome), and muscular rigidity, described as “lead pipe” in neuroleptic malignant syndrome.
- Treatment of both syndromes involves reversing the pharmacologic precipitating event: stopping serotonergic agents in serotonin syndrome and discontinuing any antidopaminergic agents in neuroleptic malignant syndrome or restarting the dopaminergic agent if abrupt withdrawal is the culprit.
- Treatment of both syndromes is largely supportive.
- Cyproheptadine (a serotonin antagonist) can be considered in moderate to severe cases of serotonin syndrome in addition to the previously mentioned interventions.
- In moderate to severe cases of neuroleptic malignant syndrome, bromocriptine can be considered when the cause is antidopaminergic drugs.
- Recovery with serotonin syndrome typically is quicker, within 24 hours, compared to neuroleptic malignant syndrome, with most patients recovering in two to 14 days.
Introduction
Serotonin syndrome and neuroleptic malignant syndrome are both potentially life-threatening conditions caused by medications. They present with altered mental status, autonomic dysfunction, and neuromuscular abnormalities. Despite overlapping features, they differ in etiology, pathophysiology, clinical presentation, and treatment. Understanding their key differences and their differential diagnoses is essential in making a timely and accurate diagnosis for effective management. Prompt recognition of the syndromes, withdrawal of the offending agent, and meticulous supportive care are critical for both.
Serotonin Syndrome
Case
A 34-year-old male develops agitation, tremors, and hypertension during his hospital admission for sepsis from bacterial pneumonia. He was initially treated with vancomycin and ceftriaxone. However, his blood cultures grew vancomycin-resistant enterococci, so the antibiotic was switched to linezolid. The patient has a past medical history of anxiety and uses paroxetine. While admitted, the patient continues to receive his home medications. He denies a history of drug or alcohol use. The admitting team noted the patient developed new agitation and tremors following the change in antibiotics. On physical examination, the patient has diffuse hyperreflexia, and both legs show five beats of clonus. He is slightly flushed, has a mild tachycardia at 115 bpm, and is hypertensive at 170/90 mmHg, which is new for him. He is afebrile. Laboratory tests show mild leukocytosis, but otherwise he has normal renal and liver functions. What is your diagnosis and treatment plan?
Etiology
Serotonin (5-hydroxytryptamine, 5-HT) is a monoamine neurotransmitter with very important roles in both the peripheral and central nervous systems (CNS). About 90% to 95% of serotonin is synthesized in the gastrointestinal system by enterochromaffin cells, while a small amount is produced in the CNS.1 Since serotonin is unable to cross the blood-brain barrier, a small amount is made in the raphe nuclei of the brainstem.1,2 In the CNS, serotonin is a modulator of excitatory neurotransmission and is notably responsible for the regulation of affect, sleep, appetite, aggression, pain perception, motor control, and cardiorespiratory function.2,3 In the peripheral nervous system (PNS), serotonin works to stimulate smooth muscle as well as to promote platelet aggregation.1 Among its many roles, serotonin also is the important precursor for melatonin.2
Serotonin is produced when tryptophan hydroxylase converts L-tryptophan into serotonin. After production, serotonin is stored in presynaptic vesicles in neurons and in platelets (8%). When neurons are stimulated, these vesicles undergo exocytosis in the synaptic cleft. Serotonin then stimulates post-synaptic receptors (5-HT receptors 1-7). Subsequently, serotonin is retaken into the presynaptic cell, where it is metabolized by monoamine oxidase (MAO) into 5-hydroxyindoleacetic acid (5-HIAA).
Serotonin syndrome is an excess of serotonergic activity in the body via over-stimulation of 5-HT receptors. This can occur from increased synthesis, inhibition of reuptake, inhibition of metabolism, or breakdown of serotonin; increased activation of post-synaptic receptors, or increased serotonin release from presynaptic neurons.1,3 Clinical findings can range broadly and include some level of autonomic hyperactivity, neuromuscular abnormalities, and mental status changes.4 Although serotonin syndrome can occur with the introduction of a single serotonergic agent in a susceptible person, or in overdose of a single agent, it more often occurs and to a more severe extent when two or more serotonergic agents with different mechanisms are used at the same time.4 Table 1 summarizes the types of medications with serotonin activity.
As the use of antidepressants increases in the United States, toxic exposures to selective serotonin reuptake inhibitors (SSRIs) and other serotonergic antidepressants also rise, highlighting the need for rapid recognition of serotonin syndrome and appropriate treatment by physicians.1,5 Although the overall incidence of serotonin syndrome is rare, one case series found that 14% to 16% of patients presenting with SSRI overdose developed serotonin toxicity.6-8 An unpublished retrospective chart review from a single hospital identified 60 cases of confirmed serotonin syndrome between 2017 and 2021 where the diagnosis appears to have been missed.9 The syndrome occurs in all ages, with no gender preference. According to the Toxic Exposure Surveillance System (TESS), there was an 18% increase in cases related to toxic exposures of SSRIs and an 8% increase in related deaths in 2016 compared to 2002.1
Table 1. Serotonergic Agents |
Selective Serotonin Reuptake Inhibitors (SSRIs)
Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs)
Serotonin Modulators and Stimulators (SMSs)
Serotonin Antagonists and Reuptake Inhibitors (SARIs)
Tricyclic Antidepressants (TCAs)
Monoamine Oxidase Inhibitors (MAOIs)
5-HT Receptor Agonists (Triptans)
5-HT3 Receptor Antagonists (in conjunction with other serotonergic agents)
Atypical Antipsychotics
Opioid Analgesics with Serotonergic Activity
Illicit Drugs with Serotonergic Activity
Medications that Inhibit the Metabolism of Serotonergic Drugs (CYP2D6 and CYP3A4)10
Miscellaneous
|
MDMA: 3,4-methylenedioxymethamphetamine Adapted from: Brown CH. Drug-induced serotonin syndrome. US Pharm. 2010;35(11):HS-16-HS-21. |
Pathophysiology
The mechanisms described previously lead to excessive stimulation of the post-synaptic serotonin receptor, especially the 5HT-2A receptor subtype. Manifestations of excess serotonergic activity can be divided into three categories, autonomic, neuromuscular, and mental status changes, and will vary depending on the severity.4 It is important to distinguish between CNS and PNS signs and symptoms of serotonin syndrome. Specifically, CNS manifestations of serotonin syndrome revolve around mental status changes. In mild cases, anxiety can be seen, while in moderate and severe cases, agitation and confusion are apparent, respectively.1,4
The more severe cases of serotonin syndrome typically are caused by the synergistic interactions that occur with the use of two or more serotonergic drugs at the same time. Particularly dangerous combinations include an SSRI with a monoamine oxidase inhibitor (MAOI) or an SSRI with the commonly used migraine medication class known as the triptans. Both SSRIs and MAOIs increase the levels of serotonin. Specifically, SSRIs block serotonin reuptake and MAOIs block the monoamine oxidase enzyme responsible for serotonin breakdown.11 The combination of SSRIs and triptans causes serotonin syndrome in a slightly different manner compared to SSRIs and MAOIs. While SSRIs increase serotonin levels specifically, triptans act directly on serotonin receptors in an agonistic manner.1
Diagnosis
The diagnosis of serotonin syndrome is a clinical one. A thorough medication history must be taken, including information such as dose changes and medication additions. The use of supplements, over-the-counter (OTC) medications, and illicit drugs also is important, since some have an effect on the serotonergic system. Serotonin syndrome typically develops within six to 24 hours of exposure to a serotonergic agent, but the onset may be faster (within just a few hours) when precipitated by parenteral or high-potency agents.
The physical examination shows the triad of mental status changes (anxiety, agitation, delirium), autonomic hyperactivity (tachycardia, hypertension, hyperthermia, dilated pupils, flushed skin, diaphoresis, increased bowel sounds), and neuromuscular changes (akathisia, tremor, hyperreflexia, clonus). Notably, the tremors and hyperreflexia are more prominent in the lower extremities.6 (See Table 2.)
Table 2. Clinical Features and Severity of Serotonin Syndrome |
Mild Toxicity
Moderate Toxicity
Severe Toxicity
|
GCS: Glasgow Coma Scale Adapted with permission from: Lynch J, Hennessy A. Serotonin syndrome. IAEM Guidelines 2024. April 2024. https://iaem.ie/professional/clinical-guidelines/ |
Laboratory testing is not required for the diagnosis, but it often shows an elevated white blood cell (WBC) count, an elevated creatinine kinase (CK) level, and a low serum bicarbonate level.
Since serotonin syndrome is a diagnosis of exclusion and has a broad differential diagnosis, clinicians should consider brain imaging when indicated. In cases of ingestions, self-harm, or overdoses, clinicians should consider ordering acetaminophen (APAP) and salicylate (ASA) serum concentrations and other drug levels as indicated. Because patients often have cardiovascular instability, an electrocardiogram (ECG) often is useful and might give clues to specific toxins involved.12
There are several published diagnostic criteria for serotonin syndrome. Sternbach and colleagues published a set of criteria in 1998, and Radomski also published criteria.13,14 However, the most widely used criteria are the Hunter Toxicity Criteria Decision Rules, which are 84% sensitive and 97% specific. The Hunter criteria include exposure to a serotonergic agent, with either recent increases in dosing, changes in the metabolism, overdoses, or the use of two drugs that have different activities in the serotonergic system. The patient must at least have one of the following symptoms: fever, spontaneous or inducible clonus, tremor, agitation, diaphoresis, or hyperreflexia. Most online medical resources list the required elements of the Hunter criteria.
Management
The most important first step is to immediately identify and discontinue all serotonergic agents.15 The rest of the management relies on meticulous supportive care, which includes the following:4
- Control agitation. This is very important, since agitated patients can rapidly develop rhabdomyolysis and can injure themselves or others. Intravenous (IV) benzodiazepines are the preferred agents.16
- Manage vital signs. Many patients will display variable changes in heart rate and blood pressure, which should be managed with agents that have a short onset of action and short duration, and are easy to titrate. For acute hypertension, esmolol, nicardipine, and nitroprusside are good choices. For hypotension refractory to fluids, epinephrine, norepinephrine, and phenylephrine are some of the preferred agents.4,6
- Manage hyperthermia. This is achieved mostly by controlling agitation, but it also might require sedation, intubation, and external cooling. Importantly, acetaminophen will not be effective in lowering the patient’s temperature.
The use of serotonin antagonists is considered after all of the previously mentioned interventions have been started. Cyproheptadine (a 5-HT1A and 2A antagonist) is the preferred agent in this class.17-19 The recommended doses are 12 mg orally (PO) and then 2 mg PO every two hours until there is an adequate clinical response. Cyproheptadine is only available as pills, so if the patient is unable to swallow, the pills must be crushed and administered by nasogastric tube (NGT). Although there are no randomized controlled trials supporting its use, retrospective and case-based evidence supports cyproheptadine as a reasonable treatment option in moderate to severe cases of serotonin syndrome.20 If available, consultation with a medical toxicologist or through the local poison control center can help guide the management of these rare and often complex cases. (See Figure 1.)
Figure 1. Algorithm for the Management of Serotonin Syndrome |
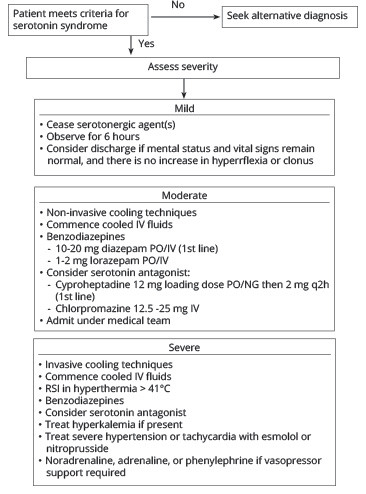 |
IV: intravenous; PO: per os; NG: nasogastric; RSI: rapid sequence intubation Adapted with permission from: Lynch J, Hennessy A. Serotonin syndrome. IAEM Guidelines 2024. April 2024. https://iaem.ie/professional/clinical-guidelines/ |
Differential Diagnosis
The differential of serotonin syndrome is broad.
Neuroleptic malignant syndrome: Serotonin syndrome and neuroleptic malignant syndrome have some overlapping clinical features. However, neuroleptic malignant syndrome, which will be discussed in depth in the following section, has slower clinical onset (on the order of days to weeks), and patients tend to instead have a slow neuromuscular response with predominant rigidity. Neuroleptic malignant syndrome also resolves more slowly (one to two weeks) than serotonin syndrome.2
Malignant hyperthermia (MH): This condition is caused by exposure to halogenated volatile anesthetics and to depolarizing muscular relaxants (succinylcholine). Its clinical hallmarks are rapid onset of severe muscle rigidity and hyperthermia.21 Up to 60% of patients have a mutation in the ryanodine receptor RYR1 of the sarcoplasmic reticulum. Rapid resolution occurs with dantrolene therapy, with 2.5 mg/kg IV bolus, which can repeated every 10 minutes.21
Anticholinergic toxicity: This occurs after exposure to anticholinergic medications. Patients will present with dry mouth, absent bowel sounds, and urinary retention. Some serotonergic medications also can be anticholinergic (for example, tricyclic antidepressants [TCAs] and paroxetine), and patients therefore can present with a mixed toxidrome. Physostigmine is the antidote and will rapidly clear the symptoms.
Other types of delirium: Delirium from infectious causes (encephalitis) and from drug withdrawal (ethanol, sedative-hypnotics) can present in an almost identical manner to serotonin syndrome.
Table 3 summarizes most relevant conditions that should be considered in the differential diagnosis of both serotonin syndrome and neuroleptic malignant syndrome.
Table 3. Differential Diagnosis for Serotonin Syndrome (SS) and Neuroleptic Malignant Syndrome (NMS) |
|||
Condition |
Similarities |
Differences |
SS or NMS or Both |
Neuroleptic malignant syndrome (NMS) |
Hyperthermia, autonomic instability, altered mental status, muscle rigidity |
Develops over days; lead-pipe rigidity (vs. clonus/hyperreflexia in serotonin syndrome); associated with dopamine antagonist use |
SS |
Malignant hyperthermia |
Hyperthermia, muscle rigidity |
Triggered by anesthesia (e.g., succinylcholine, volatile anesthetics); genetic predisposition |
Both |
Anticholinergic toxicity |
Hyperthermia, altered mental status |
Dry skin/mucous membranes, mydriasis without clonus, hyperreflexia Develops urinary retention and decreased bowel sounds |
Both |
Sympathomimetic toxicity |
Agitation, hyperthermia, tachycardia, hypertension |
No clonus or hyperreflexia; related to drugs such as cocaine and amphetamines No history of serotonergic agents in conjunction with certain drugs No history of anti-dopaminergic agent use |
Both |
Infectious causes (e.g., sepsis, meningitis, encephalitis) |
Fever, altered mental status |
Infection source, leukocytosis, no neuromuscular hyperactivity (e.g., clonus, hyperreflexia) Lumbar puncture is diagnostic for meningitis and encephalitis |
Both |
Delirium tremens and severe ethanol withdrawal |
Autonomic instability, agitation, tremors. Delirium, hallucinations |
History of ethanol use disorder with a recent decrease or cessation. History of alcohol withdrawal, hallucinations |
Both |
Heat stroke |
Hyperthermia, altered mental status, autonomic dysregulation. |
Environmental exposure to heat; lack of serotonergic drug/medication history or clonus Lack of rigidity |
Both |
(continued) |
|||
Table 3. Differential Diagnosis for Serotonin Syndrome (SS) and Neuroleptic Malignant Syndrome (NMS) (continued) |
|||
Condition |
Similarities |
Differences |
SS or NMS or Both |
Malignant catatonia (MC) |
Presents very similarly, with fever, rigidity, akinesia, and altered mental status |
In MC, the hyperthermia occurs during the prodrome of agitation. Up to 80% of patients rapidly respond to rapid administration of lorazepam, which is at times called the “lorazepam challenge.”44,49 |
NMS |
Drug-induced hyperpyrexia (salicylates and dinitrophenol [DNP]) |
Agitation, hyperthermia diaphoresis |
History of use of salicylates or DNP No clonus or rigidity |
SS |
Baclofen, gamma hydroxybutyrate (GHB) or carisoprodol withdrawal50 |
Autonomic dysregulation, agitation |
History of baclofen, GHB, or carisoprodol chronic use |
Both |
Thyrotoxicosis |
Autonomic dysregulation, diaphoresis, agitation |
Elevated thyroid hormone activity. Unexplained weight loss, dysrhythmias, tremor No clonus or rigidity Diagnosis confirmed by thyroid function tests (TFTs) |
Both |
Lithium toxicity |
Autonomic instability Altered mentation |
Has more hyperreflexia and clonus Diagnosis is confirmed by serum lithium concentration |
Both |
Status epilepticus and non-convulsive status epilepticus |
Tachycardia, altered mentation |
No hyperreflexia or clonus Diagnosis confirmed by electroencephalogram |
NMS |
Deep brain stimulator (DBS) sudden withdrawal |
Results in a sudden depletion of dopamine |
History of Parkinson’s disease and having a DBS |
NMS |
Pheochromo-cytoma and paragangliomas |
Tachycardia, fever, diaphoresis, hypertension |
Paroxysmal symptoms No rigidity or clonus Diagnosis confirmed by elevated serum or urine catecholamines |
Both |
Tetanus infection |
Tachycardia, fever |
No hyperreflexia Inducible spasms Positive spatula test Opisthotonus Recent wound and lack of vaccination |
Both |
Disposition
Patients with mild cases of serotonergic excess can be treated in the emergency department (ED) and placed in observation. Patients generally improve quickly, but the overall clinical course depends on the half-life of the agent and the underlying reason for the serotonin excess. More severe cases will require intensive care unit (ICU) admission. Intentional ingestions all must have a psychiatric evaluation.
If the patient is to be discharged from the ED, the risk vs. benefit of restarting the agent(s) must be considered. This might require involvement of a medical toxicologist, a psychiatrist, and/or a pharmacist. In all cases, a careful medication reconciliation must be done to avoid potential future events, especially when more than one agent was involved. When available, use the electronic health record and best practice alerts for medication interactions.
Finally, when prescribing serotonergic agents, educate patients about potential drug interactions and adverse events.
Special Populations for Serotonin Excess
Table 4 describes special populations at higher risk of developing serotonergic excess and serotonin syndrome.
Table 4. Populations at Higher Risk of Serotonin Syndrome |
Pediatric Patients
Geriatric Patients
Pregnant Patients
Patients with Psychiatric Illness
Intensive Care Unit or Critically Ill Patients
SSRIs: selective serotonin reuptake inhibitors; ADHD: attention deficit hyperactivity disorder; SNRIs: serotonin and norepinephrine reuptake inhibitors; MAOIs: monoamine oxidase inhibitors |
Case Conclusion
What is your diagnosis and treatment plan?
The diagnosis for this patient is serotonin syndrome. Based on the Hunter criteria, the patient is taking multiple serotonergic agents with inducible clonus, tremors, hyperreflexia, and agitation. It was triggered by his continued use of paroxetine with the addition of linezolid in the intensive care unit. Toxicology was consulted, and the following treatment plan was initiated: immediate discontinuation of all serotonergic agents; sedation with IV benzodiazepines; and administration of IV fluids. The patient remained afebrile, so cooling measures were not required. One dose of cyproheptadine was given.
The patient was monitored for complications, including rhabdomyolysis, renal failure, and electrolyte derangements such as hyperkalemia, and hyperthermia.
The symptoms improved within 24 hours of diagnosis identification. The patient was discharged after 72 hours and was scheduled to follow up with his psychiatrist to resume his paroxetine.
Neuroleptic Malignant Syndrome
Case
A 42-year-old male with a past medical history of schizophrenia, diagnosed 15 years ago, presents to the ED with high-grade fever, muscle stiffness, altered mental status, and sweating. Usually, he uses risperidone 2 mg orally per day. Ten days ago, the patient was started on haloperidol intramuscular (IM) injections (10 mg/day) because of an episode of psychotic agitation. He was doing well initially on the new medication. However, over the past two days, he developed increasing muscle rigidity and confusion and stopped eating. The family noticed he was sweating profusely and appeared very stiff. The night before, he also developed a fever.
In the ED, his vital signs are recorded as: temperature 40.1°C (104.2°F), heart rate 122 bpm, blood pressure 160/98 mmHg, respiratory rate 24/min, and O2 saturation 95% on room air. On physical examination, he is diaphoretic, confused, and minimally responsive. He displays a generalized “lead pipe” rigidity but has no other focal neurological deficits. There is no meningismus. He has no signs of trauma. Laboratory findings include a WBC count of 15,000/mm³, a CK of 8,200 U/L, mild transaminitis, and a serum creatinine of 2.0 mg/dL. His electrolytes are as follows: Na 136 mEq/L, K 4.3 mEq/L, CO2 20, and Cl 110 mEq/L. His urinalysis shows myoglobinuria. His urine toxicology screen is negative. How do you diagnose his condition? What puts him at risk for neuroleptic malignant syndrome?
Etiology
Neuroleptic malignant syndrome is a rare, idiosyncratic, life-threatening condition that results from a relative dopamine deficiency. It requires prompt recognition and treatment to prevent morbidity and mortality among those experiencing it. Prior to current awareness, its mortality was up to 25%, although most recently it is reported to be about 11.6% with increasing knowledge about the condition and its management.22,23 The development of newer anti-dopaminergic drugs has affected the prevalence as well as the morbidity and mortality from neuroleptic malignant syndrome. It is estimated today that neuroleptic malignant syndrome develops in somewhere between 0.01% to 3.2% of those taking the medications implicated in this syndrome.22
Dopamine plays a critical role in both movement regulation as well as multiple roles in the brain, such as reward, attention, learning, sleep, mood, and attention.24 There are six known dopamine neural pathways. Regarding motor function, the nigrostriatal pathway is the most important. In this pathway, dopamine is produced by cells in the substantia nigra, ventral tegmental area, and the hypothalamus and released into the striatum, the prefrontal cortex, and the nucleus accumbens.24
Pathophysiology
The pathophysiology of neuroleptic malignant syndrome is not clearly understood but generally is thought to be dopamine dysregulation in the basal ganglia and hypothalamus. Neuroleptic malignant syndrome is strongly linked to the blockade of dopamine 2 (D2) receptors, causing a disruption in dopamine signaling and a relative state of dopamine deficiency.25 Neuroleptic malignant syndrome is precipitated by dopamine receptor antagonists (antipsychotics) or by the sudden withdrawal from dopaminergic agents.26,27 Dopamine deficiency in the basal ganglia results in muscle rigidity; deficiency in the hypothalamus results in dysautonomia; and deficiency in the reticular activating system results in alterations of consciousness.22,28 Typically, the onset of neuroleptic malignant syndrome is within one to two weeks from the start of treatment or after drug adjustment. The condition was first described in the medical literature in 1956 by Frank Ayd and termed “fatal hyperpyrexia.”29
Neuroleptic malignant syndrome caused by dopamine antagonists: The older, also called “typical,” antipsychotics are the most common drugs implicated in neuroleptic malignant syndrome, with chlorpromazine being responsible for the first reported case in 1956. These drugs are potent dopamine antagonists.22,30 Other common typical antipsychotics that may lead to neuroleptic malignant syndrome include haloperidol and fluphenazine.22 The newer, also called “atypical,” antipsychotics such as clozapine, olanzapine, quetiapine, risperidone, and ziprasidone also have been responsible for cases of neuroleptic malignant syndrome because of their dopamine antagonism. Other frequently used medications such as metoclopramide, droperidol, prochlorperazine, and promethazine also block dopamine receptors and can lead to neuroleptic malignant syndrome.22 Lithium, a commonly used mood stabilizer, does not have known dopamine antagonism properties, but it is associated with neuroleptic malignant syndrome, especially when used in conjunction with the antipsychotics mentioned earlier. This likely is caused by a synergistic toxicity and not a direct cause.31 Table 5 lists all the drugs implicated in neuroleptic malignant syndrome.
Table 5. Agents Implicated in Neuroleptic Malignant Syndrome |
First-Generation (Typical) Antipsychotics
Second-Generation (Atypical) Antipsychotics
Dopamine Blockers Used for Nausea and Gastroparesis
Dopamine Drug Withdrawal
Other Agents
|
Neuroleptic malignant syndrome caused by abrupt discontinuation of dopaminergic drugs: Discontinuation of dopaminergic medications also may result in neuroleptic malignant syndrome. The most common example of this is with medications used to treat the parkinsonian diseases, such as amantadine and levodopa. It is crucial to remember that neuroleptic malignant syndrome is not only seen with the rapid discontinuation or dose reduction of these medications but also with the abrupt switching of one anti-Parkinson medication for another.22
Diagnosis
Most cases of neuroleptic malignant syndrome occur in younger patients, although this might just reflect when patients are first exposed to these medications.33 The exception is when neuroleptic malignant syndrome is caused by withdrawal from dopaminergic agents, since these agents are more commonly used in the older adult population. Men outnumber women in a 2:1 ratio. There have been familial clustering, suggesting some genetic predisposition.
Other populations at risk include those with dementia-induced psychosis, those with neurocognitive disorders, and patients with intellectual disabilities, since there are off-label uses of these anti-dopaminergic drugs for these conditions.34,35
Clinicians should obtain a thorough history that addresses risk factors for neuroleptic malignant syndrome (see Table 6) and that also includes potential alternative diagnoses, such as thyroid issues, medical problems, substance use disorder, recent exertion, and recent medical procedures (contrast agents, neuromuscular blockers, halogenated anesthetics). Table 3 describes the differential diagnoses that must be considered for both serotonin syndrome and neuroleptic malignant syndrome.
Table 6. Risk Factors for Neuroleptic Malignant Syndrome36,37 |
|
Physical Examination
The clinical presentation is fever, seen in about 87% of patients; altered mentation, seen in up to 70% of cases; rigidity (a hallmark symptom); and autonomic instability, which is the least frequent category of symptoms.41,42
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition, Text Revision (DSM-V-TR),43 has published diagnostic criteria for neuroleptic malignant syndrome. The symptoms are divided into major and minor symptoms:
Major symptoms (all are required):
- Muscle rigidity (“lead pipe”);
- Hyperthermia (> 38.0°C or 100.4°F), measured on at least two separate occasions, orally (hyperthermia [> 38.0°C] is a required feature in the DSM-V-TR criteria, but clinically, temperatures > 38.5°C to 40°C are more commonly seen in neuroleptic malignant syndrome);
- Diaphoresis;
- Exposure to a dopamine antagonist within 72 hours preceding the symptomatology.
Minor symptoms (at least two are required):
- Autonomic dysfunction: tachycardia, hypertonia, sialorrhea, urinary incontinence, pallor, tachypnea, dyspnea;
- Mental status changes: Altered mental status, delirium, stupor, coma;
- Motor changes: tremor, akinesia, dystonia, myoclonia, trismus, dysarthria, dysphagia;
- Laboratory findings: leukocytosis, elevated CK, elevated myoglobin, elevated creatinine, metabolic acidosis.
Gurrera and colleagues developed an expert consensus on the diagnosis of neuroleptic malignant syndrome and subsequently validated it.44 A cutoff of 74 points (out of a potential maximum score of 100) showed 69.6% sensitivity and 90.7% specificity.
The symptom or finding is listed with the points indicated in parentheses:
- Administration of a new agent or an increase in an existing dopamine antagonist or withdrawal of a dopamine agonist in the prior 72 hours (20 points);
- Hyperthermia, as measured orally > 100.4°F or > 38°C on at least two occasions (18 points);
- Muscle rigidity (17 points);
- Altered mental status, typically reduced or fluctuating level of consciousness (13 points);
- Rhabdomyolysis identified using a CK elevation at least four times the upper reference range (10 points);
- Sympathetic nervous system lability, with at least two of the below (10 points);
- Systolic or diastolic blood pressure elevation > 25% above baseline;
- Fluctuation in systolic or diastolic blood pressure > 20% systolic during the past 24 hours;
- Diaphoresis;
- Urinary incontinence (10 points);
- Hypermetabolic state with heart rate increase > 25% above baseline and respiratory rate increase > 50% above baseline (5 points);
• No other toxic, metabolic, infectious, or neurologic causes identified (7 points).44
Testing
Laboratory testing is not required to diagnose neuroleptic malignant syndrome. However, laboratory tests and imaging often help exclude other potential diagnoses. Because of muscle contraction and agitation, the CK is elevated, often rising above 1,000 IU/L. Elevated WBC counts, as high as 40,000 cells/microL, have been reported. Other testing might be needed to exclude other conditions resulting in fever and altered mentation, such as specific drug levels, urine for myoglobin, brain imaging, and lumbar puncture.45
Differential Diagnosis
As with serotonin syndrome, the differential diagnosis of neuroleptic malignant syndrome is broad and includes many conditions, such as serotonin syndrome, malignant hyperthermia, and malignant catatonia. Withdrawal from both ethanol and other sedative-hypnotics also can present similarly.46 The broad differential diagnoses for both SS and neuroleptic malignant syndrome are discussed in Table 3.47
Management
There are no robust evidence-based treatment protocols, so most recommendations for the management of neuroleptic malignant syndrome are based on expert consensus.45,48 Similar to serotonin syndrome, initial steps must include discontinuation of the involved agent(s) and avoidance of any anti-dopaminergic agents. If the neuroleptic malignant syndrome is the result of abrupt dopaminergic drug withdrawal, clinicians must immediately restart the anti-Parkinson medication.
Supportive management also is the cornerstone of neuroleptic malignant syndrome management. Clinicians must address hyperthermia with cooling measures. Rhabdomyolysis is managed with fluids and prevention of muscle contractions with sedatives. Avoid nephrotoxic agents, since there is a high risk of renal failure. Identify, manage, and correct any significant electrolyte derangements.
As with serotonin syndrome, manage agitation with IV benzodiazepines. Benzodiazepines also result in some muscle relaxation, which also will help patients with neuroleptic malignant syndrome. The preferred agent is lorazepam 2 mg IV, with repeated doses as needed.
For moderate to severe cases of neuroleptic malignant syndrome, consider specific pharmacologic management. If available, consult with a clinical toxicologist, since these cases often are complex.
Bromocriptine (a dopaminergic agent) is the preferred agent if the neuroleptic malignant syndrome is caused by anti-dopaminergic drugs. The dose is 2.5 mg PO q8-12h, up to 45 mg/day, Bromocriptine has case report-level evidence only, but its pharmacology should result in improved dopaminergic transmission and, thus, clinical improvement. Continue treating for 10 days after neuroleptic malignant syndrome resolution and then taper off gradually.45,49
Amantadine is the preferred agent if the neuroleptic malignant syndrome is the result of anti-Parkinson drug withdrawal, or when bromocriptine is contraindicated. The usual dose is 100 mg PO q8-12h, with an increase up to 200 mg PO q12h.50
For severe neuroleptic malignant syndrome cases, consider adding dantrolene (a direct-acting muscle relaxer) as a second-line agent. Dantrolene reduces rigidity and, therefore, heat production. The usual dosing is 1 mg/kg to 2.5 mg/kg IV bolus followed by 10 mg/kg/day as an infusion. Separate doses of 1 mg/kg to 2.5 mg/kg IV q6-8h also are used. After the patient is stable, the route can be switched to oral and continued for several days before weaning.45,49
Electroconvulsive therapy (ECT) has been used in cases refractory to pharmacological intervention.51
Disposition
Patients with neuroleptic malignant syndrome are best managed in the ICU. These patients can develop myoglobinuria and renal failure from rhabdomyolysis, disseminated intravascular coagulation, and multi-organ failure. Some patients can develop venous thromboembolism from systemic inflammation. Takotsubo cardiomyopathy has been described as well.45 The recovery from neuroleptic malignant syndrome is slower than from serotonin syndrome, with most patients recovering in two to 14 days. A toxicology consultation is strongly recommended.
Case Conclusion
The patient was started on haloperidol intramuscular injections daily for 10 days for new psychotic agitation, and these high doses put him at higher risk of neuroleptic malignant syndrome. He developed hyperthermia, severe “lead pipe” rigidity, autonomic instability, and altered mentation. This, along with the associated elevated CK, leukocytosis, and myoglobinuria, meet the diagnostic criteria of neuroleptic malignant syndrome. Toxicology was consulted and agreed with the diagnosis.
The management included immediate discontinuation of haloperidol. The patient received aggressive cooling measures, IV fluids, and IV benzodiazepines for the agitation and rigidity. The patient also received IV dantrolene and oral bromocriptine. The patient improved after this management. Intubation and paralysis were considered if rigidity and hyperthermia did not improve, but they were not required. The patient’s rigidity improved and completely resolved over six days. The CK and creatinine levels normalized. The patient returned to his baseline mental status at seven days. His psychiatric treatment was revised to a low-dose atypical antipsychotic after full recovery.
Conclusion
Both serotonin syndrome and neuroleptic malignant syndrome present with mental status changes, abnormal muscle movements, and altered vital signs. Careful attention to the pharmacologic history and the physical findings is necessary to discriminate between the two. When uncertain or with patients with severe symptoms and signs, toxicologic consultation is recommended.
Monique Graf, MD, is Attending Physician, Baylor Scott & White All Saints Medical Center, Assistant Professor, TCU Burnett School of Medicine, Fort Worth, TX.
Amy Young, MD, is Associate Professor, Department of Emergency Medicine, UT Southwestern, Dallas.
Fernando Benitez, MD, is Professor, Department of Emergency Medicine, UT Southwestern Medical Center, Dallas.
Larissa Velez, MD, is Associate Dean for Graduate Medical Education, Professor and Vice Chair for Education, Michael P. Wainscott Professorship in Emergency Medicine, Department of Emergency Medicine, UT Southwestern Medical Center, Dallas.
References
1. Simon LV, Torrico TJ, Keenaghan M. Serotonin Syndrome. In: StatPearls [Internet]. StatPearls Publishing. March 2, 2024.
2. Mills KC. Serotonin syndrome. A clinical update. Crit Care Clin. 1997;13(4):763-783.
3. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366.
4. Scotton WJ, Hill LJ, Williams AC, Barnes NM. Serotonin syndrome: Pathophysiology, clinical features, management, and potential future directions. Int J Tryptophan Res. 2019;12:1178646919873925.
5. Mojtabai R. Increase in antidepressant medication in the US adult population between 1990 and 2003. Psychother Psychosom. 2008;77(2):83-92.
6. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
7. Chiew AL, Buckley NA. The serotonin toxidrome: Shortfalls of current diagnostic criteria for related syndromes. Clin Toxicol (Phila). 2022;60(2):143-158.
8. Isbister GK, Bowe SJ, Dawson A, Whyte IM. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol. 2004;42(3):277-285.
9. Stapczynski JS. Personal communication with Will Heise, MD. May 5, 2025.
10. Mitchell PB. Drug interactions of clinical significance with selective serotonin reuptake inhibitors. Drug Saf. 1997;17(6):390-406.
11. Sub Laban T, Saadabadi A. Monoamine oxidase inhibitors (MAOI). In: StatPearls. StatPearls Publishing. 2025.
12. Yates C, Manini AF. Utility of the electrocardiogram in drug overdose and poisoning: Theoretical considerations and clinical implications. Curr Cardiol Rev. 2012;8(2):137-151.
13. Hegerl U, Bottlender R, Gallinat J, et al. The serotonin syndrome scale: First results on validity. Eur Arch Psychiatry Clin Neurosci. 1998;248(2):96-103.
14. Radomski JW, Dursun SM, Reveley MA, Kutcher SP. An exploratory approach to the serotonin syndrome: An update of clinical phenomenology and revised diagnostic criteria. Med Hypotheses. 2000;55(3):218-224.
15. Martin TG. Serotonin syndrome. Ann Emerg Med. 1996;28(5):520-526.
16. Nisijima K, Shioda K, Yoshino T, et al. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem Int. 2003;43(2):155-164.
17. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
18. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
19. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331(15):1021-1022.
20. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
21. Ali SZ, Taguchi A, Rosenberg H. Malignant hyperthermia. Best Pract Res Clin Anaesthesiol. 2003;17(4):519-533.
22. Simon LV, Hashmi HF, Callahan AL. Neuroleptic Malignant Syndrome. In: StatPearls. StatPearls Publishing. 2025 .
23. Shalev A, Hermesh H, Munitz H. Mortality from neuroleptic malignant syndrome. J Clin Psychiatry. 1989;50(1):18-25.
24. Juárez Olguín H, Calderón Guzmán D, Hernández García E, Barragán Mejía G. The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxid Med Cell Longev. 2016;2016:9730467.
25. Sweileh WM. Neuroleptic malignant syndrome and serotonin syndrome: A comparative bibliometric analysis. Orphanet J Rare Dis. 2024;19(1):221.
26. Henderson VW, Wooten GF. Neuroleptic malignant syndrome: A pathogenetic role for dopamine receptor blockade? Neurology. 1981;31(2):132-137.
27. Wijdicks EFM, Ropper AH. Neuroleptic malignant syndrome. N Engl J Med. 2024;391(12):1130-1138.
28. Oruch R, Pryme IF, Engelsen BA, Lund A. Neuroleptic malignant syndrome: An easily overlooked neurologic emergency. Neuropsychiatr Dis Treat. 2017;13:161-175.
29. Ayd FJ Jr. Fatal hyperpyrexia during chlorpromazine therapy. J Clin Exp Psychopathol. 1956;17(2):189-192.
30. Berman BD. Neuroleptic malignant syndrome: A review for neurohospitalists. Neurohospitalist. 2011;1(1):41-47.
31. Argyriou AA, Drakoulongona O, Karanasios P, et al. Lithium-induced fatal neuroleptic malignant syndrome in a patient not being concomitantly treated with commonly offending agents. J Pain Symptom Manage. 2012;44(6):e4-e6.
32. Desai D, Gupta K, Kumar R, Biswas A. Levosulpiride-induced neuroleptic malignant syndrome in rheumatoid arthritis. BMJ Case Rep. 2018;2018:bcr2018224679.
33. Keck PE Jr, Pope HG Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. A case-control study. Arch Gen Psychiatry. 1989;46(10):914-918.
34. Hefner G, Wolff J, Toto S, et al. Off-label use of antidepressants, antipsychotics, and mood-stabilizers in psychiatry. J Neural Transm (Vienna). 2022;129(11):1353-1365.
35. Yunusa I, Rashi N, Demos GN, et al. Comparative outcomes of commonly used off-label atypical antipsychotics in the treatment of dementia-related psychosis: A network meta-analysis. Adv Ther. 2022;39(5):1993-2008.
36. Berardi D, Amore M, Keck PE Jr, et al. Clinical and pharmacologic risk factors for neuroleptic malignant syndrome: A case-control study. Biol Psychiatry. 1998;44(8):748-754.
37. Guinart D, Taipale H, Rubio JM, et al. Risk factors, incidence, and outcomes of neuroleptic malignant syndrome on long-acting injectable vs oral antipsychotics in a nationwide schizophrenia cohort. Schizophr Bull. 2021;47(6):1621-1630.
38. Drews JD, Christopher A, Evans DC. Neuroleptic malignant syndrome in the trauma intensive care unit: Diagnosis and management of a rare disease in a challenging population. Int J Crit Illn Inj Sci. 2017;7(2):119-121.
39. Login IS, Cronin MJ, MacLeod RM. Neuroleptic malignant syndrome caused by dopamine depleting drugs. Neurology. 1982;32(2):218-219.
40. Ossemann M, Sindic CJ, Laterre C. Tetrabenazine as a cause of neuroleptic malignant syndrome. Mov Disord. 1996;11(1):95.
41. Tormoehlen LM, Rusyniak DE. Neuroleptic malignant syndrome and serotonin syndrome. Handb Clin Neurol. 2018;157:663-675.
42. Rajan S, Kaas B, Moukheiber E. Movement disorders emergencies. Semin Neurol. 2019;39(1):125-136.
43. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th-TR ed. American Psychiatric Press; 2022
44. Gurrera RJ, Mortillaro G, Velamoor V, Caroff SN. A validation study of the international consensus diagnostic criterai for neuroleptic malignant syndrome. J Clin Psychopharmacol. 2017;37:67-71.
45. Kuhlwilm L, Schönfeldt-Lecuona C, Gahr M, et al. The neuroleptic malignant syndrome — a systematic case series analysis focusing on therapy regimes and outcome. Acta Psychiatr Scand. 2020;142(3):233-241.
46. Paul G, Parshotam GL, Garg R. Carisoprodol withdrawal syndrome resembling neuroleptic malignant syndrome: Diagnostic dilemma. J Anaesthesiol Clin Pharmacol. 2016;32(3):387-388.
47. Orsolini L, Volpe U. Expert guidance on the differential diagnosis of neuroleptic malignant syndrome. Expert Rev Neurother. 2025;25(2):125-132.
48. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
49. Schönfeldt-Lecuona C, Kuhlwilm L, Cronemeyer M, et al. Treatment of the neuroleptic malignant syndrome in international therapy guidelines: A comparative analysis. Pharmacopsychiatry. 2020;53(2):51-59.
50. Apetauerova D, Patel PA, Burns JD, Lerner DP. Movement disorder emergencies. Neurol Clin. 2021;39(2):615-630.
51. Morcos N, Rosinski A, Maixner DF. Electroconvulsive therapy for neuroleptic malignant syndrome: A case series. J ECT. 2019;35(4):225-230.