Diabetic Emergencies
September 15, 2025
By Jessica Zhen, MD, and Jordan Hickey, MD
Executive Summary
- Diabetes continues to increase across all age groups, making diabetic emergencies a frequent challenge in the emergency department.
- Both diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic syndrome (HHS) can occur in type 1 or type 2 diabetes, with infection being the most frequent precipitating factor.
- Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are strongly associated with euglycemic DKA, which requires high suspicion given near-normal glucose levels.
- Symptoms of diabetic emergencies often are vague; rapid bedside tests such as point-of-care glucose and capnography (to assess for acidosis) are valuable diagnostic tools.
- Management of DKA focuses on fluids, electrolyte repletion, insulin therapy, and reversal of acidosis, although with important nuances.
- In HHS, the serum sodium and osmolality play a greater role in guiding fluid therapy, which typically develops more gradually than DKA.
- Intubation in DKA requires caution and should be avoided, if possible, since mechanical ventilation may disrupt the respiratory compensation for metabolic acidosis.
- Most patients with hyperglycemic emergencies will require admission for intravenous infusions, although mild DKA may be treated with subcutaneous insulin; many hypoglycemic patients can be discharged if stable and without ongoing risks.
- Early recognition, efficient evaluation, and tailored management are critical, since DKA, HHS, euglycemic DKA, and severe hypoglycemia all can be fatal without prompt intervention.
Introduction
According to the Centers for Disease Control and Prevention (CDC), 38.4 million people in the United States have diabetes, 8.7 million are undiagnosed, and 97.6 million adults have prediabetes.1 With such a high prevalence and rising incidence, patients with diabetes presenting to the emergency department (ED) for associated complications, as well as unrelated issues, are inevitably common.
Although diabetes affects various organ systems and complicates other disease processes, pure diabetic emergencies include diabetic ketoacidosis (DKA), hyperosmolar hyperglycemic syndrome (HHS), euglycemic diabetic ketoacidosis (EDKA), and severe hypoglycemia. These emergencies often are precipitated in a patient with known diabetes but frequently can be the initial presentation in someone with undiagnosed diabetes. It is essential for ED providers to understand the pathophysiology, clinical features, workup, and management of these conditions, since they can be fatal, as they often were before the availability of insulin.
This article will discuss the epidemiology, etiology, pathophysiology, clinical features, differential diagnoses, workup, management principles, and disposition of these diabetic emergencies: DKA, HHS, EDKA, and hypoglycemia. A focus on distinguishing characteristics and nuances of each emergency will be discussed.
Epidemiology
Approximately 537 million adults worldwide are living with diabetes, and that number is expected to climb to 783 million by the year 2045.2 Three out of four people with diabetes are residents of low- to middle-income countries. Because of a significant increase in incidence and prevalence in the past two decades, diabetes became the ninth leading cause of death globally in 2019 and resulted in the largest rise in male deaths among the top 10 causes of death.
These data mirror the impact that diabetes has in the United States, with diabetes currently the eighth leading cause of death. The number of youth and young adults with diabetes and prediabetes is particularly astonishing. From 2001 to 2017, the number of people younger than age 20 years living with type 1 diabetes (DM1) increased by 45%, and the number living with type 2 diabetes (DM2) grew by 95%.3 While DM1 remains more common in white youth, DM2 is more common and rising more rapidly in racial and ethnic minority groups, most notably Black, Hispanic, and American Indian. Similar rising numbers are seen with prediabetes: one in five adolescents (ages 12-18 years) and one in four young adults (ages 19-34 years) in the United States, with a higher prevalence in males and those with obesity.4
The seemingly unstoppable rise in diabetes and prediabetes, particularly in youth and young adults, means more long-term health consequences and, ultimately, more healthcare costs. Acute complications also will continue to increase, contributing to more ED visits. For both DM1 and DM2, those who experience DKA/HHS are more likely to be Black or Hispanic and of lower income.5 Interestingly, among inner city young adults, there have been parallels between hemoglobin A1c (HbA1c) and some of these demographics; young adults admitted for DKA/HHS had severely uncontrolled diabetes with HbA1c > 12% more associated with being Black and uninsured.6
Although DKA more commonly is associated with DM1, it can occur under extreme stress in DM2. It is more common in those younger than age 65 years, while HHS is seen more often in those older than 65 years. From 2000-2009, DKA hospitalization rates decreased, but this trend has reversed, with a steady increase in DKA hospitalization rates from 2009 to 2014 at an average annual rate of 6.3%.7 In a large commercially insured population with DM1, the incidence of DKA was 55.5 per 1,000 person-years between 2007-2019.8 Nationwide mortality rates for DKA decreased from 0.51% in 2003 to 0.33% in 2014 from analysis of the National Inpatient Sample.9 An analysis of the same data source for 2017 found a slight uptick in mortality to 0.38%, with higher rates in males, Blacks, and older adult patients.10
There is less population-based data for DKA and HHS in DM2; however, the rate of admissions for HHS is lower than that for DKA, but the mortality rate is significantly higher, ranging from 10% to 20%.7
EDKA is even more rare, with an incidence between 2.6% to 3.2% of DKA admissions.11 However, this likely is an underrepresentation of the disease process because of the lack of consensus for a definitive cutoff of serum glucose. The three most common situations associated with EDKA are pregnancy, prolonged fasting, and the use of sodium-glucose cotransporter-2 (SGLT-2) inhibitors for treating diabetes.11,12 Analysis of the Food and Drug Administration’s (FDA) adverse event reporting system on DKA incidence with SGLT-2 inhibitors found a seven-fold increased risk for DKA, and around two-thirds of the reported DKA cases were euglycemic.12 The risk is higher in patients with significant insulin insufficiency or DM1 (up to 9%), and, therefore, the FDA does not recommend SGLT-2 inhibitors for DM1.12
Hypoglycemia, on the other hand, is much more common, with data from the 2024 National Diabetes Statistics Reports indicating 202,000 ED visits for hypoglycemia, and other sources indicating that it is one of the most common acute metabolic events leading to hospitalization in the diabetic population.1,13
Etiology
Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic Syndrome
It was long believed that DKA was an emergency complication limited to those with DM1, and HHS was unique to patients with DM2. However, while these are the associations that are commonly recognized in clinical practice, now it is well documented that there is more overlap between these two entities than previously thought.14 For instance, DKA often can be the first presentation of previously undiagnosed DM1 or DM2. Most commonly, DKA is the result of a complete or absolute deficiency of insulin, a characteristic usually found with DM1, but studies have shown that it is important to keep in mind that patients presenting with DKA ultimately may be diagnosed with DM2.15,16 Per some reports, it is possible that this number of eventual DM2 diagnoses could even be as high as one-third of all cases.17
While DKA can be the initial indication of underlying diabetes, it more commonly presents in those with a known diagnosis.18 Similarly, while the classic presentation of HHS involves an older patient with a known diagnosis of DM2, there are a growing number of cases of HHS in people with DM1 and as the initial representation of both classes of diabetes mellitus.19 Additionally, it is possible to see characteristics of both disease processes present at one time, although this article will largely continue to refer to them as separate entities.20 Nonetheless, while it is being found that presentations and associations with underlying disease may be more heterogeneous than previously thought, precipitating factors for these two diabetic emergencies share many similarities, and it is important to remember to investigate and treat the underlying trigger.
For both HHS and DKA, the most prevailing inciting circumstances in adults are infection and omission of either insulin dosing or antidiabetic medications.19-22 However, although these are the most common, an initial broad approach must be used, since the potential inciting causes are numerous, and most of the associated morbidity and mortality come from them rather than from the pathophysiology of the diabetic emergency itself. Other precipitating causes that should be considered in DKA and HHS if associated signs and/or symptoms are present are listed in Table 1.14,16,19-25
Table 1. Potential Precipitants of Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic Syndrome |
Diabetic Ketoacidosis
Hyperosmolar Hyperglycemic Syndrome
|
Euglycemic Diabetic Ketoacidosis
As noted earlier, the three most common precipitating events for EDKA are pregnancy, the use of SGLT-2 inhibitors, and a fasting state where insulin is being used. Other possible inciting factors that should be considered include chronic alcohol use, substance use (primarily cocaine), liver disease, infections, recent bariatric surgery, and cerebral or myocardial infarction.20,26
Hypoglycemia
As with hyperglycemic emergencies, hypoglycemia usually occurs in the setting of an underlying trigger. It can be spontaneous, although this is a rarer occurrence. Rather, it usually is seen in patients with underlying diabetes mellitus and frequently is a complication of treatment. (See Table 2.)
Table 2. Medications that Can Increase the Risk of Hypoglycemia16,28-30 |
|
The risk of hypoglycemia increases with age, since autonomic failure begins to occur, renal clearance of insulin declines, oral intake decreases, and increased polypharmacy makes patients more susceptible.16,27,28 Other possible etiologies of hypoglycemia include means by which sensitivity to insulin is increased, such as in exercise, fasting, and weight loss.
Hypoglycemia also can be induced in situations where gluconeogenesis and glycogenolysis are impeded, such as in hepatic failure, kidney injury, and with alcohol consumption. Additionally, although it may be largely out of the scope of ED management, it is important to keep in mind that hypoglycemia may be the presenting feature in metabolism dysregulation, particularly in the setting of endocrine disorders (e.g., Addison’s disease, panhypopituitarism, growth hormone deficiency, or hypothyroidism).16,28,29
Pathophysiology
Diabetes
Simply put, diabetes results from excessive glucose in the blood because of a lack of insulin or inability to use insulin properly, ultimately leading to widespread pathology if excessive levels are not treated. Insulin is the main anabolic hormone of the body and is released from beta cells in the islet of Langerhans of the pancreas in response to an increase in blood glucose. It promotes uptake of glucose from the blood into the liver, fat, and muscle, where it can be used immediately for energy or converted into glycogen and triglycerides for energy stores. Opposing insulin is glucagon, the main catabolic hormone of the body that is released from the alpha cells of the pancreas. When the serum blood glucose is low, glucagon is released, triggering gluconeogenesis and glycogenolysis in the liver and lipolysis in adipose to increase glucose and fatty acids to be used as energy. The counterbalancing interaction of insulin and glucagon regulate each other to restore and maintain normoglycemia.31
In DM1, there is autoimmune destruction of the insulin-producing pancreatic beta cells, so no insulin is made. This occurs in genetically susceptible individuals and likely is triggered by a variety of factors, such as viral infections or changes in the gut microbiome. There is no identified association between diet or obesity in DM1. After the inciting event, it can take months to years to manifest hyperglycemia because of the large number of beta cells that need to be destroyed to affect glucose metabolism.
DM1 typically is diagnosed in childhood, with two age peaks, 4 to 7 years and 10 to 14 years of age. The presence of autoantibodies in DM1 — termed DM1A — along with the progressive destruction of beta cells allows three stages to be defined for DM1A: stage 1, in which autoantibodies present but the patient is normoglycemic; stage 2, autoantibodies present and the patient is dysglycemic but without symptoms; and stage 3, autoantibodies, dysglycemia, and symptoms are present. There is a much smaller subgroup of DM1 — DM1B — that presents the same as DM1A, but upon testing, no autoantibodies are found.32
In contrast, DM2 results from insulin resistance and impaired insulin secretion. Normally, the liver responds to insulin by decreasing glucose release and instead using it to build glycogen stores. However, when it is resistant to insulin, it inappropriately releases glucose into the blood, contributing to hyperglycemia. Although the mechanism is not understood, obesity, particularly central adipose, and lack of exercise are heavily linked to the development of DM2. The impaired insulin secretion likely is twofold from genetic influences as well glucotoxicity where hyperglycemia has a toxic effect on pancreatic beta cells, resulting in a destructive cycle.33 While DM2 is classically called adult-onset diabetes, this has proven to be a misnomer, since the incidence and prevalence among children and young adults is climbing because of rising obesity at an earlier age.
According to the American Diabetes Association (ADA), to be diagnosed with diabetes, one of the following criteria must be met:34
- HbA1C ≥ 6.5%;
- Eight-plus-hour fasting plasma glucose ≥ 126 mg/dL (7.0 mmol/L);
- Two-hour plasma glucose ≥ 200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test;
- In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥ 200 mg/dL (11.1 mmol/L); the most common way DM is identified in the ED.
Diabetic Ketoacidosis
The defining features of DKA include hyperglycemia (> 200 mg/dL), acidosis (pH < 7.3), and ketonemia/ketonuria (beta-hydroxybutyrate ≥ 3.0 mmol/L or urine ketones ≥ 2+).35 The serum bicarbonate levels typically are low (< 18 mEq/L), and the anion gap is elevated. These metabolic changes ultimately occur because of insulin deficiency and/or resistance, which allows for excess counterregulatory hormone activity, including glucagon, catecholamines, and cortisol. (See Figure 1.) Despite elevated serum glucose, without the negative feedback signal of insulin, the body believes it is in a state of hypoglycemia.
Figure 1. Pathophysiology of Diabetic Ketoacidosis |
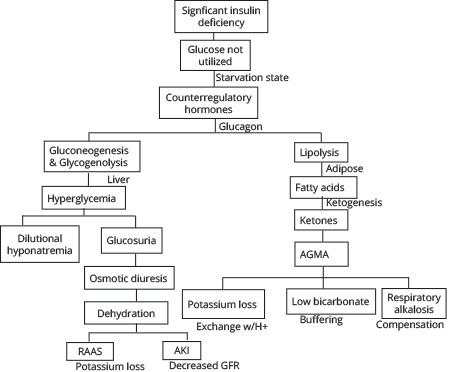 |
AGMA: anion gap metabolic acidosis; RAAS: renin-angiotensin-aldosterone system; AKI: acute kidney injury; GFR: glomerular filtration rate |
The liver performs glycogenolysis and gluconeogenesis to increase serum glucose, contributing to hyperglycemia. This excess blood glucose increases the osmolality of the blood and, to autoregulate, fluid shifts from in cells to the vasculature. Ultimately, the kidneys see this excess fluid and glucose and attempt to compensate via the process of osmotic diuresis, leading to dehydration.21 Over time, though, the dehydration impairs the kidney by affecting the glomerular filtration rate (GFR) and can lead to acute kidney injury (AKI). There is a resulting hypovolemic hyponatremia because of the dilutional effect of fluid shifts on sodium in the vasculature; traditionally, for every 100 mg/dL increase in glucose above normal (100 mg/dL), there is an estimated 1.6 mEq/L decrease in serum sodium.36 However, this may be an underrepresentation of the degree of dilutional hyponatremia, as Hillier showed, especially when there is significant hyperglycemia > 400 mg/dL, and a larger correction factor of 2.4 mEq/L for every 100 mg/dL above normal may be more accurate.37
The absence of insulin results in glucagon-induced lipolysis of triglycerides to produce free fatty acids. These undergo beta oxidation and ketogenesis to become the ketone bodies acetoacetic acid and beta hydroxybutyric acid, which serve as an energy source in starved states.21 However, as their names suggest, they have a low pH and cause the elevated anion gap metabolic acidosis. To maintain physiologic pH, the body uses the bicarbonate buffering system (CO2 + H2O D H2CO3 D HCO3- + H+), accounting for the low bicarbonate levels seen. However, in DKA, this mechanism cannot fully compensate, and often patients are noted to be hyperventilating to blow off CO2, ultimately countering the metabolic acidosis with a respiratory alkalosis.
Although many electrolytes are affected in DKA, potassium is one of the most significant, since it has important effects on many organs, including cardiovascular functioning. Dehydration triggers the renin-angiotensin-aldosterone system (RAAS), which stimulates sodium and chloride resorption in the tubules of the kidneys to retain water in exchange for potassium that is lost. As the metabolic acidosis worsens, hydrogen ions are shifted intracellularly in exchange for potassium ions intravascularly, yet these are lost via osmotic diuresis. In addition, there often is gastrointestinal loss of potassium in the form of vomiting and decreased oral intake. Although serum values may be normal or even hyperkalemic, the total body potassium is reduced because of these factors, with total body deficits commonly 300 mEq to 600 mEq.21
Hyperosmolar Hyperglycemic Syndrome
A significantly elevated serum glucose > 600 mg/dL, profound dehydration, and hyperosmolality > 320 mOsm/kg without significant ketosis, acidosis, or bicarbonate derangement is the metabolic picture of HHS. Although the hyperglycemia and dehydration may be very similar to DKA, the disease process differs. This is because in HHS the insulin deficiency is less severe, but insulin resistance persists and may be acutely worse. In addition, one of the main contributing factors to the disease pathophysiology is that the patient cannot hydrate adequately. HHS occurs more in the older adult population, who may have an impaired thirst mechanism or debility that inhibits easy access to water. This propagates a worsening cycle of hyperosmolality, osmotic diuresis, and dehydration, ultimately leading to the average 9 L water deficit in these patients.
The presence of some insulin is sufficient enough in HHS to prevent lipolysis, so ketones are minimally, if not at all, produced.6,19 Therefore, as opposed to DKA, there is little effect on serum acid-base status. Unfortunately, the concentration of insulin required to suppress lipolysis is only one-tenth that required to promote glucose utilization, so while ketone formation is blocked, there is not enough insulin to promote glucose utilization or prevent gluconeogenesis and glycogenolysis in the liver.6
The hyperglycemia in HHS is more extreme than in DKA for two reasons: ketoacidosis in DKA causes earlier symptoms than the hyperosmolality of HHS, so DKA patients present sooner, and patients with DKA tend to be younger with a better renal function and more capacity to excrete glucose to at least initially slow the rise of hyperglycemia.6 The extreme hyperglycemia and glucosuria in HHS leads to an osmotic diuresis of largely electrolyte-free urine, causing a greater degree of dehydration and increased effective plasma osmolality compared with DKA.19 This is exacerbated by patients failing to compensate for the water loss, leading to a worsening cycle of hyperglycemia, hyperosmolality (mainly sodium), and hypovolemia. (See Figure 2.)
Figure 2. Pathophysiology of Hyperosmolar Hyperglycemic Syndrome |
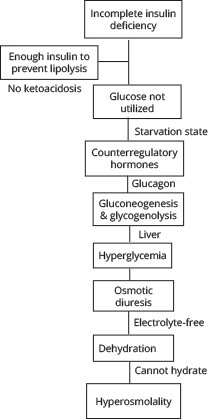 |
Similar to DKA, serum potassium levels in HHS may be normal to high initially, but total body stores are low. The potassium deficit in HHS usually is greater than in DKA because of hyperosmolality; with water moving out of the cell, potassium is both dragged out of the cell and also passively moves out as the intracellular concentration rises with the loss of water. In addition, insulin normally promotes potassium uptake by cells, but with the insulin deficiency and resistance, it cannot aid in this process. Osmotic diuresis, as in DKA, continually flushes extracellular potassium out of the body.
Euglycemic Diabetic Ketoacidosis
As the name suggests, EDKA is characterized by euglycemia — a relatively normal blood glucose (< 250 mg/dL) — but with the rest of the findings of DKA, including metabolic acidosis with pH < 7.3, low serum bicarbonate < 18 mEq/L, and the presence of ketones.11 A carbohydrate deficit, rather than the insulin deficiency or resistance, is the main factor contributing to this pathophysiology.11 Ultimately, in EDKA, a fasting state causes decreased serum insulin and depletes the liver’s glycogen stores so glycogenolysis and gluconeogenesis cannot compensate, and the hyperglycemia of DKA is not seen. However, because of the lack of carbohydrates and, therefore, energy, counterregulatory hormones still are released, and glucagon triggers ketogenesis, producing the other findings typical of DKA.11,12 (See Figure 3.) Just as in DKA, dehydration occurs from decreased oral intake, vomiting, and osmotic diuresis, and only increases the counterregulatory hormones worsening the lipolysis and ketogenesis.
Figure 3. Pathophysiology of Euglycemic Diabetic Ketoacidosis |
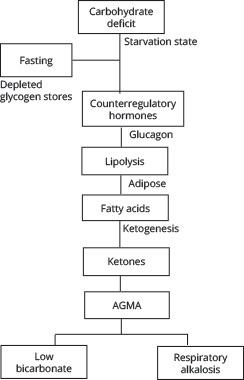 |
AGMA: anion gap metabolic acidosis |
As previously mentioned, EDKA has three main precipitating causes. SGLT-2 inhibitors are a class of medication to treat diabetes by preventing reabsorption of glucose in the proximal convoluted tubule of the kidney, producing glucosuria to decrease glucose levels in the body. However, this creates a state of carbohydrate deficit and volume depletion, the two components that promote glucagon release leading to an increase in the glucagon/insulin ratio and ketogenesis with euglycemia.11,12
Normal physiologic changes in pregnancy include mechanisms that enhance nutrient storage in the mother and shunt glucose to the fetus and placenta, producing a state of carbohydrate deficit and insulin resistance in the mother.11,12 In addition, because of the increased levels of progesterone, there is a natural respiratory alkalosis in pregnancy, and the body compensates with bicarbonate loss in the urine. Therefore, the stage is set for any trigger, commonly pregnancy-associated vomiting, to cause EKDA, since there already is a carbohydrate deficit, increased counterregulatory hormones, and a diminished ability to buffer acidosis, and ketogenesis and metabolic acidosis occur faster during pregnancy than when not pregnant and at lower blood sugar levels.12
Partially treated DKA will cause EDKA as well. In states of fasting or illness, there is a carbohydrate deficit, but if someone taking insulin does not adjust for this, it will keep serum glucose low and glycogen stores depleted and will prevent gluconeogenesis, but lipolysis and ketogenesis will continue for needed energy.12
Hypoglycemia
Hypoglycemia, simply defined, is a state of low blood sugar. As the brain relies on glucose as its main source of energy, reductions in serum glucose can impair neurological function. Usually, the body has several mechanisms to prevent hypoglycemia, and it is a rare occurrence in healthy individuals.
The ADA defines hypoglycemia as any low level of serum glucose (with or without symptoms) that exposes the patient to harm.34 It often is defined as serum glucose < 70 mg/dL, which is the lower limit of the physiologic fasting nondiabetic range. However, more recently, ≤ 54 mg/dL was deemed clinically important biochemical hypoglycemia, since it does not occur in nondiabetics under physiologic conditions and has identifiable immediate and long-term harm.38
In both DM1 and DM2, over time, there is a progressive loss of the pancreatic beta cells that release insulin, which prevents paracrine crosstalk to the glucagon-secreting alpha cells, leading to impaired release during hypoglycemia.39 The sympathetic defense also is impaired over time in what has been described as hypoglycemic-associated autonomic failure; episodes of hypoglycemia reset the threshold for epinephrine release to a lower glucose level.39 The most common cause of hypoglycemia in patients with diabetes is the medications used to treat diabetes. Usually this is associated with mismanagement of medication, such as continuing to take exogenous insulin during fasting states, skipped meals, increased activity, and illness.
Clinical Features
Diabetes
The classic symptoms of diabetes mainly are due to ongoing hyperglycemia. Once the serum glucose exceeds the renal threshold for glucose reabsorption, glucose spills into the urine. Increased blood glucose and glucosuria cause osmotic diuresis, leading to the polyuria and nocturia as well as dry skin. This ultimately leads to dehydration, hypovolemia, and reflexive polydipsia. Despite having increased hunger drive and ample amounts of glucose, people with diabetes cannot use it properly for energy, and the body turns to using other sources of fuel, including stored glucose, fat, and muscle, contributing to unintentional weight loss and fatigue.
Diabetic Ketoacidosis
As a result of the multifactorial pathophysiology of DKA, the presenting chief concerns and clinical features often can be complicated and vary based on the severity of illness. In general, DKA is thought to precipitate in an acute fashion, usually developing over hours and at most a few days. The general appearance of patients can range from relatively well-appearing and only demonstrating mild symptoms of volume loss to obtunded with impending respiratory failure. Because of the hyperglycemia and osmotic diuresis, polyuria and compensatory polydipsia may be the only symptoms present until metabolic derangements develop.
Generalized abdominal pain is one of the most common chief complaints, and it tends to correlate with the severity of acidosis.16,21,40 Other common early and often vague chief complaints to the ED include nausea, vomiting, loss of appetite, general weakness, and fatigue.16,20 Significant unintentional weight loss usually only occurs in the setting of a new diagnosis where patients have had a prolonged state of hyperglycemia.40 Osmotic diuresis and vomiting lead to volume depletion, which can present with subjective symptoms, such as lightheadedness and dry mouth, but can produce physical signs such as tachycardia, orthostatic hypotension, poor skin turgor, and dry mucous membranes.16
As ketoacidosis further develops, patients begin to exhibit tachypnea and hyperpnea to reduce pCO2, producing respiratory alkalosis to compensate for the worsening metabolic acidosis.41 Eventually, as acidosis progresses, patients will begin to experience Kussmaul respirations: rapid, deep, and labored respirations at a constant pace.41 Classically, a fruity breath odor can be detected because of the increase in acetone production.16,21,42
And as discussed previously, DKA generally is precipitated by another event or disease that needs to be addressed in management. As such, it is vital to obtain a history encompassing medication administration, glucose monitoring, and insulin pump function, if the patient has one. It also is necessary to obtain a review of systems to assess any underlying infection, ischemia, substance use, concurrent endocrine pathology, hemorrhage, possible pregnancy, or coagulopathy.16,21,23
Hyperosmolar Hyperglycemic Syndrome
In contrast to DKA, HHS tends to evolve over a longer period of time, with symptoms progressing over days to weeks.16,19,20,43 Presenting chief complaints often are general and nonspecific, such as generalized weakness, fatigue, vomiting, muscle cramps, and anorexia.16,20 Because there is a significant hyperglycemic load present, patients often will have osmotic diuresis that presents in the form of polyuria and polydipsia and often leads to profound dehydration.20
It also is common for patients to present with neurologic abnormalities, including but not limited to lethargy, confusion, delirium, visual changes, and sensory deficits.19,20 Altered mental status correlates to the magnitude of dehydration and hyperosmolality, with it usually seen in patients with a serum osmolality > 330 mOsm/kg.6,14,19 Some studies have suggested that up to 15% of patients may present with focal or generalized seizures that often are resistant to anticonvulsant agents.16,20 Evaluation of the patient commonly shows signs of significant dehydration, including dry mucous membranes, poor skin turgor, sunken eyes, tachycardia, hypotension, and hypothermia.16,19,20
Additionally, because HHS commonly is precipitated by another pathology or event, it is important to obtain as much information as possible regarding potential causes. This may be difficult at times if patients are presenting with altered mental status, but the provider should attempt to include a detailed review of medications and symptoms of infection, particularly pneumonia and urinary tract infections, since they are the most common. It also is essential to complete a review of systems to assess for any symptoms of hemorrhage, ischemia, infarction, ingestion, inflammation, or trauma.16,19,44 For instance, in contrast to DKA, because significant ketosis usually is not present in HHS, abdominal pain typically is not a presenting symptom. If abdominal pain is present, further workup is necessary because it likely is related to a precipitating cause rather than due to the pathophysiology HHS itself.19,23
Euglycemic Diabetic Ketoacidosis
Similar to DKA, EDKA presents with many of the same vague symptoms, including nausea, vomiting, anorexia, and generalized fatigue. With progression of the disease state, patients also begin to exhibit Kussmaul breathing. However, unlike DKA, polyuria and polydipsia are not as pronounced without a significant glucose load to cause these osmotic symptoms. Similarly, while often present, dehydration is not as marked in EDKA.20,26,45
It is important to note that the diagnosis of EDKA often is delayed because of the normal or near-normal levels of glucose and, subsequently, often results in a delay in management. Therefore, this diagnosis relies heavily on good history-taking skills to assess risk factors and maintaining a high suspicion for the pathology. Important topics to address during evaluation to uncover potential precipitating causes of EDKA include medication history (e.g., SGLT-2 inhibitors), fasting, alcohol intake, extensive exercise, pregnancy, history of liver disease, history of renal disease, recent surgery (e.g., bariatric surgery), history of gastroparesis, and any symptoms that could suggest an infection.12,26,45 Furthermore, it is imperative to ask patients with insulin-dependent diabetes if they administered insulin for their symptoms prior to arrival to the ED, since this could reflect a true DKA diagnosis in which treatment was self-initiated by the patient.26
Hypoglycemia
In evaluating for hypoglycemia, the presenting signs and symptoms usually are divided into neuroglycopenic or autonomic in nature. Neuroglycopenic signs and symptoms include confusion, lightheadedness, lethargy, agitation, seizures, combativeness, stroke-mimicking symptoms such as focal neurological deficits, and even coma. In response to hypoglycemia, autonomic symptoms include both adrenergic and cholinergic pathway activation. Adrenergic symptoms include palpitations, tremors, anxiety, irritability, nausea, and vomiting. Because of cholinergic activation, symptoms also may include diaphoresis, paresthesias, and hunger.16,29
Overall, in evaluation of the patient, obtaining a history directly from the patient may be difficult at times with neurologic symptoms being prevalent. However, when possible, information should be obtained regarding risk factors for hypoglycemia, including medication history, medical history (e.g., diabetes, renal disease), symptoms of serious infection, symptoms associated with hormone deficiencies (e.g., adrenal insufficiency), or drug and alcohol use.16,46,47
Whipple’s triad is a set of three conditions that must be met to define a hypoglycemic crisis: signs and symptoms of hypoglycemia are present, the measured glucose is ≤ 70 mg/dL, and the signs and symptoms of hypoglycemia resolve after blood glucose rises and returns to normal. Several categories of hypoglycemia can be defined:38
- Severe: an event requiring another person to actively administer carbohydrates, glucagon, or other resuscitation efforts. Plasma glucose may not be measured during the event, but neurological recovery attributable to the restoration of plasma glucose is sufficient to conclude hypoglycemia as the cause;
- Documented symptomatic: typical symptoms of hypoglycemia accompany a measured glucose < 70 mg/dL;
- Asymptomatic: an event without the typical symptoms of hypoglycemia but with a measured glucose < 70 mg/dL;
- Probable symptomatic: an event with typical symptoms of hypoglycemia but glucose not measured but presumably low glucose was the cause;
- Pseudohypoglycemia: an event with typical symptoms of hypoglycemia but measured glucose ≥ 70 mg/dL.
Diagnostic Studies
Most adult patients presenting the ED with DKA, HHS, or EDKA will be ill, and likely will have a known diagnosis of diabetes. Often, they will have had elevated glucose readings on home glucometers or admit to nonadherence with insulin regimens. Histories such as this streamline the diagnostic workup for hyperglycemia, acidosis, and electrolyte abnormalities. However, even in adults, especially with the rising incidence of prediabetes and diabetes, it is possible that one of these emergencies is the initial presentation of diabetes.
Confirming DKA, HHS, or EDKA with laboratory studies is only part of the puzzle; while it will help guide therapy and disposition, there often is an underlying trigger that needs to be diagnosed and addressed to fully treat the patient. These triggers may have associated metabolic derangements and organ abnormalities that also must be evaluated and monitored. Similarly, associated trauma is not uncommon, either as a trigger for the diabetic emergency or as a result, and should be considered. Most often, a broad laboratory and diagnostic workup is essential to fully diagnose, treat, and monitor these patients appropriately. (See Table 3.)
Table 3. Diagnostic Evaluation |
Essential Studies
Considerations to Identify Triggering Events
|
Diabetic Ketoacidosis
In DKA, the hyperglycemia typically is in the 300 mg/dL to 500 mg/dL range, venous blood gas (VBG) will show the metabolic acidosis with a compensatory respiratory alkalosis, and serum bicarbonate will be decreased.48 Ketones will be present on urinalysis, but serum testing specifically for beta hydroxybutyrate also should be performed for quantitative measurement. Beta hydroxybutyrate is the most common ketone and is produced in a much higher ratio than acetoacetate, a ratio that continues to increase with worsening DKA. In addition, serum beta hydroxybutyrate levels > 3.8 mmol/L have high specificity and sensitivity for the diagnosis of DKA.48
As mentioned previously, DKA is associated with several electrolyte abnormalities: pseudohyponatremia and a normal or elevated serum potassium level despite overall low total body potassium. A similar situation occurs with phosphate where the total body level is low because of insulin deficiency and metabolic acidosis, but the serum levels are normal until they fall due to intracellular shifts occurring with insulin and fluid treatment.48 Other laboratory findings include leukocytosis usually in the range of 10,000 cells/μL to 15,000 cells/μL, likely stress-induced from excess cortisol, catecholamines, and pro-inflammatory cytokines. However, if the white blood cell (WBC) count is > 25,000 cells/μL or with > 10% bands, it can suggest ongoing infection.48,49 Other laboratory findings that are common in DKA are elevated lipase despite the lack of intraabdominal pathology and elevated triglycerides. The HbA1c level can help differentiate chronic hyperglycemia of uncontrolled diabetes from acute metabolic decompensation from someone who previously had well-controlled diabetes.49
The ADA categorizes DKA in adults into one of three stages of severity:50
- Mild: blood pH mildly decreased between 7.25-7.30, serum bicarbonate decreased to 15 mmol/L to 18 mmol/L, and the patient is alert;
- Moderate: blood pH 7.00-7.25, serum bicarbonate decreased to 10 mmol/L to 15 mmol/L, and mild drowsiness may be present;
- Severe: blood pH below 7.00, serum bicarbonate decreased below 10 mmol/L, and stupor or coma may be present.
Often underused, end-tidal carbon dioxide (ETCO2) monitoring/capnography can be used to assist in the diagnosis of DKA. Typically, a VBG or arterial blood gas (ABG) is performed to confirm metabolic acidosis, which is one of the diagnostic criteria for DKA. However, these tests are reliant on laboratory turnaround. Therefore, ETCO2 can be used as a proxy for the partial pressure of carbon dioxide (PaCO2) portion of the ABG, since it has a direct relationship with arterial carbon dioxide and metabolic acidosis.51 In DKA, metabolic acidosis (decreased pH) ensues because of the presence of ketoacids and loss of bicarbonate. The body attempts to compensate by blowing off more carbon dioxide via increasing the minute ventilation to cause a respiratory alkalosis, which lowers ETCO2 measurements. Therefore, there is a linear correlation between the respiratory alkalosis seen in DKA and ETCO2. As acidosis worsens, serum pH decreases, bicarbonate decreases, and ETCO2 also decreases.48
For adult patients presenting to the ED with hyperglycemia (> 550 mg/dL) and a suspected diagnosis of DKA, studies have near 100% sensitivity in excluding the presence of metabolic acidosis with ETCO2 levels > 35 mmHg.48,51,52 In addition, the specificity nears 100% in identifying metabolic acidosis and DKA with ETCO2 levels < 21 mmHg.48,51,52 Capnography can serve as a quick, cheap, and noninvasive means to assess for metabolic acidosis in patients presenting to the ED with possible DKA. Furthermore, knowing the relationships between acidosis, bicarbonate, and ETCO2 measurements allows for easy continuous monitoring, noting clinical improvement or deterioration as well as response to treatments.
Hyperosmolar Hyperglycemic Syndrome
Profound hyperglycemia likely is one of the first and very striking features of HHS. Point-of-care (POC) testing usually will read just “high,” since serum glucose results often are > 1,000 mg/dL. The other important laboratory abnormality is the serum osmolality, which is always elevated in HHS, helping distinguish it from DKA. As discussed previously, this is because of an increased effective plasma osmolality; the substantial amount of glucose in the serum with largely electrolyte-free water output via osmotic diuresis increases the osmolality, which is exacerbated by impaired thirst mechanism and/or reduced access to water, allowing for normal or increased effective sodium and osmolality.53 HHS does not cause an osmolar gap, and if one is found, further studies should be done to find a source of the unmeasured anions. Of note, because of the significant degree of hyperglycemia, the correction factor of 2.4 mEq/L vs. 1.6 mEq/L for correction of pseudohyponatremia likely is more accurate.54 Similar to DKA, leukocytosis can be seen, as well as potassium derangements. Although it often is seen in DKA, kidney injury typically is associated with HHS.
The ADA includes the following as diagnostic features of HHS:50
- plasma glucose levels > 600 mg/dL;
- serum osmolality > 320 mOsm/kg;
- profound dehydration, up to an average of 9 L;
- serum pH > 7.30;
- serum bicarbonate > 15 mEq/L;
- absent to small ketonuria and absent to low ketonemia < 3 mmol/L;
- blood urea nitrogen (BUN) > 30 mg/dL;
- creatinine > 1.5 mg/dL;
- some alteration in consciousness.
Euglycemic Diabetic Ketoacidosis
What distinguishes EDKA from DKA is a normal glucose in the presence of the expected metabolic acidosis and ketosis; serum glucose will be < 250 mg/dL, pH will be < 7.3, serum bicarbonate will be < 18 mEq/L, and ketones will be present in blood and urine. As with the other hyperglycemic emergencies, there may be leukocytosis, but significant elevation should prompt investigation for concurrent infection. Potassium alterations occur as in DKA and HHS, with the propensity to appear normal despite overall total body depletion. Magnesium and phosphate often are low, potentially more so than in the other hyperglycemic conditions, since EDKA occurs in a starved state. On the other hand, while there may be mild hyponatremia, it generally is less severe than the pseudohyponatremia seen in profound hyperglycemic states.55
Hypoglycemia
A POC fingerstick glucose measurement will diagnose hypoglycemia rapidly. As mentioned, a level < 70 mg/dL is considered low, but < 54 mg/dL is the clinically important level. However, it still is important to get a serum glucose value via a chemistry panel test. In patients with a history of diabetes who present symptomatic with documented low glucose, it likely is due to their medications, but a thorough history, physical examination, and laboratory workup should be conducted to rule out underlying triggers.
Management
Similar to all presentations to the ED, the evaluation of hyperglycemic emergencies begins with a primary survey — airway, breathing, circulation, disability, and exposure — and intervening in these areas when necessary.
Diabetic Ketoacidosis
Even with only a brief evaluation and minimal information, such as hyperglycemia on a POC glucose test, if there is a high suspicion for DKA, management should be initiated prior to receiving confirmatory laboratory tests.61 Overall, the management of DKA has the following goals:48,56,57,58-61
- fluid resuscitation to address dehydration and to improve hemodynamic stability and tissue perfusion;
- correcting electrolyte derangements;
- correcting acidosis;
- correcting hyperglycemia;
- identifying and treating the inciting event.
As with most dynamic pathologies, frequent and methodical reevaluation of patients is key in monitoring the effectiveness of and then adjusting the next steps of treatment. This includes repeat physical examination to assess for changes (e.g., mental status, circulation, breathing status, and accurate intake and output), as well as hourly POC glucose checks and repeat laboratory tests to monitor electrolytes, kidney function, and acid-base status.62,63
Fluid Resuscitation and Continuous Intravenous Fluids
While the extent of volume depletion can vary, it is typical that patients presenting with DKA can have a fluid deficit of ~100 mL/kg, which is approximately 4 L to 6 L, but it can be as high as 10 L.56-58,61 As such, the initial step in management should be obtaining intravenous (IV) access and administering fluids to replenish intravascular volume. This will allow for perfusion of organs, improving hemodynamic stability in patients with hypotension, decreasing insulin resistance, and working to decrease the levels of serum glucose and ketone levels.48,49,57,62-64
It is largely agreed upon that IV fluid administration is one of the cornerstones of DKA management, but there has been much academic debate regarding which fluids to choose and at what rate they should be given. Many protocols are based on the most recent ADA guidelines released in 2009 that recommend the initial use of a normal saline bolus at 15 mL/kg/hr to 20 mL/kg/hr.50,57,65 However, concerns have been raised for the use of sodium chloride infusions and their risk of causing hyperchloremic metabolic acidosis, contributing to the already present acidosis and potentially prolonging its resolution.57,66,67 Several studies in the early 2010s investigated whether balanced electrolyte solutions (e.g., lactated Ringer’s) would be more beneficial in DKA resuscitation. And while they showed that balanced fluids may reduce the risk of developing hyperchloremic metabolic acidosis, no true benefit was found in the time that it took to normalize acid-base disturbances.68,69
However, more recently, a Phase II trial comparing 0.9% sodium chloride to Plasmalyte-148 (a buffered solution) demonstrated a shorter time to normalization of acid-base disturbances.70 Similarly, in 2020, a subgroup analysis was completed using two previous large randomized controlled trials that demonstrated a significant decrease in time of DKA resolution in patients who were given balanced electrolyte solutions when compared to those resuscitated with normal saline.71
That said, the most recently released society guidelines come from the Joint British Diabetes Societies in 2021, which continue to recommend normal saline as the fluid of choice in initial resuscitation.72 This is partially because of the logistical aspect that normal saline can be premixed with potassium chloride, another crucial aspect in the treatment of DKA that will be discussed later, rather than separately administering balanced solutions and potassium replacement. Nonetheless, despite current guidelines, the use of buffered solutions over normal saline may be considered on a case-by-case basis and as more research is completed, future guidelines may shift to recommending their use.
If the patient is hemodynamically unstable, they may require further fluid boluses until they begin to reach a more euvolemic state and blood pressure has stabilized.2 Otherwise, after the initial bolus, the adult patient can be switched to a maintenance infusion rate at 250 mL/hr to 500 mL/hr. In hyponatremic patients, it may be best to use normal saline, whereas if the patient is eunatremic or hypernatremic, half-normal saline can be used.48,57,58,61
In most cases, glucose will normalize quicker than ketoacidosis. As such, because insulin administration is needed to resolve the ketoacidosis, its time of required use likely will exceed the time that it takes for glucose to normalize. Because of this, once glucose levels begin to approach 200 mg/dL to 250 mg/dL (different sources vary on this cutoff level), dextrose must be added to the IV fluids to allow for continued insulin administration and avoid hypoglycemia. This can be accomplished by switching fluids to 5% dextrose in half-normal saline.14,48,57,63,67
The approach to IV fluid administration described earlier often is referred to as a “one-bag” system, since the fluids are completely altered based on glucose levels, and only one IV is running at a time. Alternatively, there is the “two-bag” system that currently is not used regularly in the adult population but also is an option for management. Rather than entirely switching to IV fluids with a set amount of dextrose once a patient’s blood glucose levels reach a certain range, the two-bag system works to keep insulin and fluid infusion rates constant while titrating the amount of dextrose administration to blood glucose levels. In this method, the patient will have a consistent level of IV insulin being infused. Simultaneously, the patient would have two bags of IV fluids being administered, each with different compositions (one is half-normal saline and the other is 10% dextrose with half-normal saline), and the rate at which each is titrated is based on glucose levels to keep an overall constant rate of fluid infusion.60,73,74
This approach to DKA management has largely been studied only in pediatric populations, but several studies have demonstrated benefits that may be translatable to the adult population. For instance, various studies have shown a quicker bicarbonate, ketone, and pH correction; shorter stays in the pediatric intensive care unit (PICU); and a reduction in the length of time spent receiving insulin therapy.75-77 More recently, several studies have evaluated the two-bag system in adults. In 2017, a retrospective study showed the use of the two-bag system led to quicker closure of the anion gap and blood glucose levels when compared to the one-bag system.74 Similarly, a before-and-after implementation in an ED setting of a two-bag method protocol demonstrated a quicker resolution of acidosis, decreased duration of IV insulin, and decreased need for hospitalization or intensive care unit (ICU) admissions.28 Lastly, a retrospective review was published in 2019 that, although it did not show the same benefits as previously mentioned in the adult studies, it did support the safety of the two-bag approach and that it is an appropriate alternative to the one-bag approach.78
The last consideration in the administration of fluids in DKA management is the risk of iatrogenic fluid overload. While fluid resuscitation is key in the treatment of DKA, it should be used with caution in patients with heart failure, those who are dialysis dependent, and patients with advanced liver disease. It is imperative to monitor the overall status of these patients closely to help guide fluid resuscitation (e.g., vitals, improved signs of dehydration, urine output, and cardiac function). These patients may benefit more from starting with smaller boluses of IV fluids (250 mL to 500 mL) and close monitoring of changes to clinical status.50,65,67,79 Some experts recommend the use of ultrasound, primarily the views of the inferior vena cava and cardiac windows, to better determine a patient’s volume status and adjust fluid administration accordingly.67 The administration of IV fluids in DKA management is summarized in Figure 4.
Figure 4. Approach to Intravenous Fluids in the Management of Diabetic Ketoacidosis |
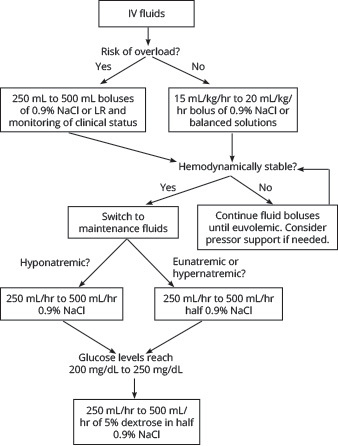 |
IV: intravenous; NaCl: sodium chloride; LR: lactated Ringer’s |
Electrolyte Replacement
As previously mentioned, it is not uncommon for a patient with DKA to have multiple associated electrolyte abnormalities, so it is important to check their levels frequently throughout treatment, initially every two hours, and then the timing can be spread out once the patient becomes more stable.48 The most consequential of these irregularities is potassium. Because of extracellular shifts secondary to acidosis, lack of insulin, and volume depletion, patients may present with normal to high levels of serum potassium even though they have a total body potassium deficit. The treatments initiated in DKA management to address these abnormalities can exacerbate this deficit, making potassium replacement an important factor to address.14,57,61-63,65 Potassium levels should be obtained promptly and assessed prior to starting insulin therapy. Typically, if a patient’s initial potassium is > 5.2 mEq/L, insulin can be started without prior potassium replacement. However, if the patient’s potassium is 3.3 mEq/L to 5.2 mEq/L, insulin can be started with concurrent potassium replacement. Lastly, if the patient’s potassium is < 3.3 mEq/L, insulin must be held until adequate potassium replacement has occurred.14,57
Recent reviews even suggest a more conservative approach to potassium replacement, proposing that insulin not be initiated until potassium levels reach 3.5 mEq/L.48 Per ADA recommendations, the goal on repeated potassium serum monitoring should be to maintain a level of 4 mEq/L to 5 mEq/L. To accomplish this, 20 mEq to 30 mEq of potassium should be given for every 1 L of fluids administered.50,65 Alternatively, the more recent British guidelines recommend 40 mEq of potassium for each liter of fluids administered when potassium levels are < 5.5 mEq/L.72 (See Figure 5.)
Figure 5. Approach to Potassium Replacement in Diabetic Ketoacidosis Management Based on ADA Guidelines |
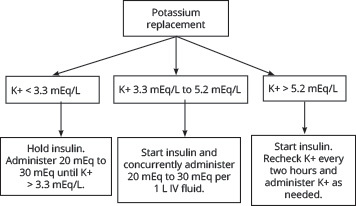 |
ADA: American Diabetes Association; K+: potassium; IV: intravenous |
Table 4. General Principles of Fluid Therapy in Hyperosmolar Hyperglycemic Syndrome54 |
Inadequate fluid administration is suggested if:
Continue with 0.9% normal saline (NS) if:
Switch to 0.45% NS or lactated Ringer’s (LR) if:
Hold fluids if:
If severe overcorrection > 8 mOsm/kg per hour, administer D50 boluses. |
Hypomagnesemia also may result as a consequence of osmotic diuresis. Therefore, magnesium also should be monitored closely and replaced, since it will aid in potassium absorption and also can help mitigate the risk of dysrhythmias if the patient is profoundly hypokalemic.48 One to two grams of IV magnesium sulfate can be given over an hour for replacement as needed, with a goal level of > 2 mg/dL.48,57
Phosphate replacement also could be considered if a patient’s serum levels are severely depleted (e.g., < 1 mEq/dL, cardiac dysfunction, respiratory depression, skeletal muscle weakness, seizures); however, it is not routinely recommended. There is a risk of inducing hypocalcemia with phosphate repletion, so it should be used cautiously. If needed, 20 mEq to 30 mEq of potassium phosphate can be added to 1 L of replacement fluid.14,48,50,65
Insulin Administration
As stated before, the goal of insulin therapy is not only to decrease serum glucose levels by promoting peripheral glucose metabolism, but also to inhibit ketone production and subsequently resolve the ketoacidosis. However, the resolution of glucose levels usually occurs prior to the resolution of acidosis, so the ultimate goal of insulin therapy should be the closure of the anion gap.57,60,65 After the initial fluid resuscitation is complete, and the patient’s potassium is within appropriate levels, insulin administration should be initiated. Because of its easy titration and short half-life, it is recommended to start patients on continuous IV regular insulin at an infusion rate of 0.1 units/kg to 0.14 units/kg per hour without a preceding loading dose.50,57,65,72 At this rate, it is expected that blood glucose levels will decrease by 50 mg/dL to 75 mg/dL per hour.57,58
If this decrease is not seen within the first hour of its infusion, the insulin rate can be doubled, and it also is necessary to ensure that other measures of treatment — fluids and electrolyte replacement — are optimized.48,57,58 As previously discussed, once glucose levels reach 250 mg/dL, the patient must start to receive dextrose in fluids, since the insulin infusion should continue until the anion gap is resolved, and it is necessary to mitigate the risk of hypoglycemia. Once glucose levels are < 200 mg/dL, the insulin infusion rate can be decreased to 0.02 units/kg to 0.05 units/kg per hour.50,57,61
The insulin infusion should continue until glucose is < 200 mg/dL and two of the following qualifiers have been met:57,65
- serum bicarbonate level > 15 mEq;
- venous pH > 7.3;
- anion gap < 12 mEq/L;
- ability to tolerate oral intake.
At this point in time, the care team can initiate transition to subcutaneous (SC) insulin. The transition to SC insulin should overlap with IV therapy for about two to four hours to circumvent relapse hyperglycemia. Per the more recent British recommendations, patients also should be given either their home dose of basal insulin or started on 0.25 units/kg of short-acting insulin during DKA management to aid in preventing rebound hyperglycemia as well.14,72 Otherwise, the transition to SC insulin is going to vary on a case-by-case basis and individual institutional policy and usually will involve the help of the available diabetes specialists. While it is not always a reality, it is likely that by this point the patient has been admitted and this transition step will be managed by the inpatient team.48,56,60,72
Considering Bicarbonate
Bicarbonate administration is not routinely recommended in the management of DKA, since acidosis is treated with and usually resolves with the methods discussed earlier. Additionally, the use of bicarbonate has been associated with possible risks, such as worsened intracellular acidosis, worsening of hypokalemia, cerebral edema, increased time to acidosis resolution, paradoxical central nervous system acidosis, hypertonicity, and hypernatremia.48,49,57,61 Nonetheless, the ADA recommends the administration of sodium bicarbonate if the initial pH is < 6.9 because of the severity of acidosis and the critical nature of the patient’s pathology.50 In contrast, the British guidelines do not recommend its use in DKA patients because of potential risks and lack of evidence reporting benefits.14,72 As such, the use of bicarbonate should be used judiciously and on a case-by-case basis when needed.
Euglycemic Diabetic Ketoacidosis
Management of EDKA adheres to the same principles as that of DKA: fluid resuscitation, insulin administration, and electrolyte replacement. While the IV fluid resuscitation and electrolyte monitoring and repletion follow the same management as discussed earlier, the insulin administration for EDKA differs slightly than for DKA. Because the serum glucose is relatively normal (≤ 250 mg/dL), dextrose (5% or 10%) should be added initially to fluids to help prevent hypoglycemia and expedite clearance of ketosis.56,80,81 Similarly, it is recommended to avoid an insulin bolus and use the lower dose (0.1 unit/kg per hour) for continuous insulin infusion until the anion gap closes.56,80,81 Additionally, if the patient is taking an SGLT-2 inhibitor, it is important to discontinue this until recovery and then consult endocrinology to determine the best regimen for continued outpatient management of diabetes.
Hyperosmolar Hyperglycemic Syndrome
Similarly, while HHS is very similar to DKA and shares many of the same principles of treatment and goals, there are some key differences that affect management. This is important to know, since most protocols combine DKA and HHS, including ADA recommendations. First, because HHS occurs over a period of several days, as opposed to DKA (which develops quite rapidly), physiologic abnormalities should be corrected more slowly and controlled to prevent complications, such as cerebral edema and osmotic demyelination syndrome (ODS).54 Therefore, fluid administration, including rate and composition, depends on both the corrected serum sodium levels and serum osmolality. Beginning with normal saline (NS, 0.9% sodium chloride) boluses and infusion for immediate resuscitation in the setting of hypotension and hypovolemia is appropriate and can follow the same recommendations as for DKA discussed earlier.
However, once stabilized, the glucose, serum osmolality, and corrected sodium should be calculated and used to help guide fluids. The fluids themselves should help to decrease glucose levels at approximately 75 mg/dL to 100 mg/dL per hour. The change in serum osmolality should be kept at 3 mOsm/kg per hour or less to prevent rapid alterations that can lead to cerebral edema.54 As for the sodium, for every 100 mg/dL drop in glucose, there is a 2.4 mEq/L increase in sodium. Both serum osmolality and corrected sodium should be monitored every one to two hours during treatment. General principles for fluid therapy in HHS are listed in Table 4.
In HHS, half of the fluid deficit should be restored in the first 18-24 hours and the remainder over the next 24 hours to aid with this slow correction back to normal physiology.54
Insulin administration in HHS treats the hyperosmolality rather than ketoacidosis in DKA.54 In addition, although the glucose is significantly more elevated in HHS than in DKA, the fluid administration helps decrease both glucose and the hyperosmolality, and it is acceptable to hold insulin infusion until there is a plateau in glucose levels. For these reasons, a decreased insulin need makes sense, and proceeding without a bolus to a lower infusion dose can be effective. The recommended insulin dose is a 0.05 unit/kg to 0.1 unit/kg per hour regular insulin infusion along with the patient’s home dose of long-acting insulin or 0.3 U/kg glargine SC.54
Using protocols developed for DKA is acceptable for treating HHS, but insulin should be reduced or stopped once glucose levels reach 300 mg/dL while fluids are continued until hyperosmolality resolves.54,63 The goal in treating HHS is correction of serum glucose, electrolytes, and osmolality. (See Table 5.)
Table 5. Correction Targets in Hyperosmolar Hyperglycemic Syndrome Management7,12,37 |
|
Hypoglycemia
As previously discussed, it is important to obtain a POC glucose measurement when hypoglycemia is suspected because prompt recognition and treatment are crucial to avoid detrimental effects. Treatment is based on the patient’s level of alertness and ability to swallow.
In an asymptomatic patient who is found to be hypoglycemic on testing, treatment is straightforward. Provide a source of carbohydrates for them to eat and recheck glucose in 15 minutes. Repeat as needed and ensure proper medication dosages and patient understanding of medication administration. For a symptomatic patient who is awake and alert, similar treatment can be used with oral administration of approximately 15 g to 20 g of quickly absorbable carbohydrates (e.g., fruit juice, regular soda, sugar water, crackers, hard candies, or glucose tablets). Retest POC glucose levels after 15 minutes and again repeat as necessary. Once glucose levels are stabilized and remain ≥ 70 mg/dL, the patient should have a meal or snack with longer-acting carbohydrates to prevent recurrence of hypoglycemia. Of note, if the patient is taking an alpha glucosidase inhibitor, only pure glucose, such as glucose tablets, should be ingested. This is because the medication slows digestion of disaccharides, so other forms of carbohydrates will be less effective in acutely raising blood sugar.82
If the patient is unconscious or significantly altered, or if there is a concern for aspiration or the patient is not receiving anything by mouth for any other reason, treatment with IV dextrose should be initiated. Intravenous treatment generally consists of a bolus dose of 50 mL of 50% dextrose (D5W), typically in a prefilled syringe colloquially called D50. Glucose then should be rechecked in 15 minutes. If the patient still is hypoglycemic, these initial steps can be repeated two more times, rechecking glucose every 15 minutes.
In the case of a patient continuing to be hypoglycemic after these initial interventions, or if IV access is unable to be obtained, 1 mg of intramuscular or SC glucagon should be administered.
In addition, there are now widely available ready-to-use glucagon options that the patient may self-administer in the setting of a hypoglycemic crisis, including intranasal preparations (typically 3 mg of glucagon), and prefilled autoinjector/injectable preparations.83
In patients who require ongoing boluses, who do not return to their neurological baseline, who cannot tolerate taking medication by mouth, or who have hypoglycemia secondary to long-acting medications, an infusion of D5W or D10W should be initiated for ongoing maintenance. As always, frequent glucose monitoring is essential.
Of note, hypoglycemia related to sulfonylurea toxicity can be particularly difficult to treat, with a potentially prolonged course. Octreotide should be administered concurrently with the IV dextrose, as mentioned earlier. Octreotide is a somatostatin analog that inhibits insulin release from pancreatic beta cells. Solo treatment with IV dextrose in sulfonylurea toxicity can cause transient hyperglycemia, which triggers insulin release and resulting hypoglycemia. The octreotide helps prevent the insulin release, ultimately creating a steadier state.57,84-88 The most common dose of octreotide is 50 mcg to 100 mcg intramuscular or subcutaneous every six hours.
Additional Aspects
It is essential to initiate therapy as quickly as possible for hyperglycemic crises, since delayed care can lead to serious complications and sequelae, including seizures, coma, and organ failure. Within the first 48-72 hours, the majority of the deaths in hyperglycemic crises are due to the precipitating cause, potassium derangements, and cerebral edema.65 As previously mentioned, HHS has a higher overall mortality rate than DKA, which is even more exemplified when treatment is delayed. Acute myocardial and bowel infarction can occur secondary to prolonged hypotension in DKA, and the massive dehydration in HHS can lead to additional complications, such as stroke, pulmonary embolism, disseminated intravascular coagulation, and mesenteric vein thrombosis.65
Although some patients may present critically ill and airway, breathing, and circulation take priority, intubation should be avoided unless necessary. Because of the metabolic acidosis, minute ventilation increases to expire more CO2, thereby compensating the metabolic acidosis via a respiratory alkalosis. Any period of apnea, even if short during intubation, can cause further acidosis and elevation of CO2 levels. Following intubation, matching the minute ventilation can be difficult, and that level of compensation is difficult to achieve. Ultimately, intubation may worsen the acidosis and contribute to circulatory collapse. If intubation is absolutely required, use mask ventilation during any apnea, use measures to optimize first-pass success, use short-acting paralytics, and set a ventilator mode that lets the patient set the rate and tidal volume for hyperventilation.56
While prompt management is essential, there also are complications to be aware of from treatment. Hypoglycemia and hypokalemia are the most common adverse effects, but they can be avoided with careful monitoring.25,89 One of the most feared complications with treatment of DKA is cerebral edema, which usually develops within the initial 12 hours but can be delayed up to two to three days. It almost exclusively occurs in people younger than 20 years of age and has a significant mortality rate of 20% to 40%.25,56,89 Cerebral edema is more common with sicker presentations. The pathophysiology is not fully understood but does not appear to be related to initial osmolality or osmotic changes during management of DKA; however, some small animal studies suggest that a rapid decline in osmolality can contribute to cerebral edema in HHS, so it is recommended to keep serum glucose near 300 mg/dL during treatment.56 Symptoms are those of increased intracranial pressure, and treatment is with mannitol and/or hypertonic saline.
Disposition
Hyperglycemic Emergencies
For hyperglycemic crises, nearly all patients require admission to the hospital. Often, this will be to the ICU because of the frequency of POC glucose checks, laboratory tests, monitoring needs, and attendance to medication drips. For very mild cases that respond well to resuscitation or only require subcutaneous insulin, discharge may be possible, but only after a thorough workup, and this is best done in consultation with the patient’s endocrinologist.56
While IV insulin is the standard of care per ADA guidelines, there are some studies showing similar outcomes with the use of subcutaneous insulin in mild to moderate DKA.56 Subcutaneous shorter-acting insulin (aspart or lispro) administered every one to two hours has shown no difference in mortality, duration of treatment until correction of hyperglycemia or resolution of ketoacidosis, length of hospital stay, episodes of hypoglycemia, or total insulin dose.56,61,90,91 This potentially could reduce ICU admission and costs in mild to moderate DKA, but still requires close glucose monitoring.
Resolution of hyperglycemic emergencies is indicated by correction of dehydration and normalization of serum glucose and electrolytes. For DKA, serum glucose should be < 200 mg/dL, and additional parameters must be attained: serum bicarbonate ≥ 15 mEq/L, pH ≥ 7.3, and anion gap ≤ 12 mEq/L.65 For HHS, these criteria plus normalization of osmolality are needed.
Hypoglycemia
For hypoglycemic emergencies, admission is not as definite. As mentioned previously, if the patient is having repeat episodes of hypoglycemia, is not back to baseline despite improved serum glucose, requires multiple doses of D50, is on a dextrose drip, has other pathology, or has toxicity from long-acting medications (particularly sulfonylureas), admission is indicated. The admission unit will be based on their monitoring needs and whether continuous infusions are running.
Summary
Diabetes is rising across all age groups, ensuring that diabetic emergencies are common in the ED. Both DKA and HHS can occur in type 1 or type 2 diabetes, most often triggered by infection. SGLT-2 inhibitors are a frequent cause of EDKA, and their use should prompt diagnostic consideration. Because symptoms often are vague, point-of-care glucose checks and capnography are simple, rapid tools to aid diagnosis. While DKA and HHS share treatment goals, nuances in treatment are important to recall, since HHS fluid management is guided more so on sodium and osmolality. Most hyperglycemic emergencies require admission, although mild DKA may be treated with subcutaneous insulin and many hypoglycemic patients can be discharged if no ongoing risks exist. Prompt recognition and tailored management are essential, since DKA, EDKA, HHS, and hypoglycemia can be life-threatening. When possible, providing patient education on these diabetic emergencies is important and the ADA 2025 guidelines stress earlier recognition and prevention of hyperglycemic emergencies, including better use of monitoring and addressing social determinants.92
Jessica Zhen, MD, is Attending Emergency Medicine Physician, USAF, MC, Wright Patterson AFB, OH; Core Faculty Member, Emergency Medicine Residency WPAFB/Wright State University, Boonshoft School of Medicine, Dayton, OH.
Jordan Hickey, MD, is Instructor, Core Faculty Member, Department of Emergency Medicine, Wright State University Boonshoft School of Medicine, Dayton, OH.
References
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report. May 15, 2024. https://www.cdc.gov/diabetes/php/data-research/index.html
2. International Diabetes Federation. Diabetes around the world in 2021. IDF Diabetes Atlas. Last updated 2021. https://diabetesatlas.org
3. Centers for Disease Control and Prevention. New research uncovers concerning increases in youth living with diabetes in the U.S. Aug. 24, 2021. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/media/releases/2021/p0824-youth-diabetes.html
4. Andes LJ, Cheng YJ, Rolka BD, et al. Prevalence of prediabetes among adolescents and young adults in the United States, 2005-2016. JAMA Pediatr 2020;174:e194498.
5. McCoy RG, Galindo RJ, Swarn KS, et al. Sociodemographic, clinical, and treatment-related factors associated with hyperglycemic crises among adults with type 1 or type 2 diabetes in the US from 2014 to 2020. JAMA Netw Open 2021;4:e2123471.
6. Wolf RA, Haw JS, Paul S, et al. Hospital admissions for hyperglycemic emergencies in young adults at an inner-city hospital. Diabetes Res Clin Pract 2019;157:107869.
7. Hirsch IB, Emmet M. Diabetic ketoacidosis and hyperosmolar hyperglycemic state in adults: Epidemiology and pathogenesis. 2021. UpToDate. Updated June 23, 2021. https://www.uptodate.com/contents/diabetic-ketoacidosis-and-hyperosmolar-hyperglycemic-state-in-adults-epidemiology-and-pathogenesis
8. Li L, Andrews EB, Li X, et al. Incidence of diabetic ketoacidosis and its trends in patients with type 1 diabetes mellitus identified using a U.S. claims database, 2007-2019. J Diabetes Complications 2021;35:107932.
9. Desai D, Mehta D, Mathias P, et al. Health care utilization and burden of diabetic ketoacidosis in the U.S. over the past decade: A nationwide analysis. Diabetes Care 2018;41:1631-1638.
10. Ramphul K, Joynauth J. An update on the incidence and the burden of diabetic ketoacidosis in the U.S. Diabetes Care 2020;43:e196-e197.
11. Plewa MC, Bryant M, King-Thiele R. Euglycemic diabetic ketoacidosis. In: StatPearls [Internet]. StatPearls Publishing; updated Jan. 24, 2022.
12. Nasa P, Chaudhary S, Shrivastava PK, Singh A. Euglycemic diabetic ketoacidosis: A missed diagnosis. World J Diabetes 2021;12:514-523.
13. Simon E, Sessions D. The adult hypoglycemic patient: Tips for emergency department management. emDocs. Nov. 3, 2016. http://www.emdocs.net/adult-hypoglycemic-patient-tips-emergency-department-management/
14. Karslioglu French E, Donihi AC, Korytkowski MT. Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome: Review of acute decompensated diabetes in adult patients. BMJ 2019;65:l1114.
15. Zhong VW, Juhaeri J, Mayer-Davis EJ. Trends in hospital admission for diabetic ketoacidosis in adults with type 1 and type 2 diabetes in England, 1998-2013: A retrospective cohort study. Diabetes Care 2018;41:1870-1877.
16. Tintinalli JE, Ma OJ, Yealy DM, et al. Emergency Medicine: A Comprehensive Study Guide. 9th edition. McGraw-Hill Education; 2020.
17. Eledrisi MS, Elzouki AN. Management of diabetic ketoacidosis in adults: A narrative review. Saudi J Med Med Sci 2020;8:165-173.
18. Stratigou T, Vallianou N, Vlassopoulou B, et al. DKA cases over the last three years: Has anything changed? Diabetes Metab Syndr 2019;13:1639-1641.
19. Long B, Willis GC, Lentz S, et al. Diagnosis and management of the critically ill adult patient with hyperglycemic hyperosmolar state. J Emerg Med 2021;61:365-375.
20. Muneer M, Akbar I. 2020. Acute metabolic emergencies in diabetes: DKA, HHS and EDKA. In: Islam MS, ed. Diabetes: from Research to Clinical Practice. Springer;2020.
21. Long B, Willis GC, Lentz S, et al. Evaluation and management of the critically ill adult with diabetic ketoacidosis. J Emerg Med 2020;59:371-383.
22. Dhatariya KK. Defining and characterising diabetic ketoacidosis in adults. Diabetes Res Clin Pract 2019;155:107797.
23. Dingle HE, Slovis C. Diabetic hyperglycemic emergencies: A systematic approach. Emerg Med Pract 2020;22:1-20.
24. Stoner GD. Hyperosmolar hyperglycemic state. Am Fam Physician 2017;96:729-736.
25. Hirsch IB, Emmett M. Diabetic ketoacidosis and hyperosmolar hyperglycemic state in adults: Clinical features, evaluation, and diagnosis. UpToDate. Updated June 6, 2022. https://www.uptodate.com/contents/diabetic-ketoacidosis-and-hyperosmolar-hyperglycemic-state-in-adults-clinical-features-evaluation-and-diagnosis?search=dka&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2
26. Long B, Lentz S, Koyfman A, Gottlieb M. Euglycemic diabetic ketoacidosis: Etiologies, evaluation, and management. Am J Emerg Med 2021;44:157-160.
27. Freeman J. Management of hypoglycemia in older adults with type 2 diabetes. Postgrad Med 2019;131:241-250.
28. Lamos EM, Younk LM, Davis SN. Hypoglycemia. In: Matfin G, ed. Endocrine and Metabolic Medical Emergencies: A Clinician’s Guide. 2nd ed. John Wiley & Sons Ltd;2018:506-530.
29. Mathew P, Thoppil D. Hypoglycemia. In: StatPearls [Internet]. StatPearls Publishing; Jan. 4, 2022.
30. Blumer I, Clement M. Type 2 diabetes, hypoglycemia, and basal insulins: Ongoing challenges. Clin Ther 2017;39:S1-S11.
31. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev 2018;98:2133-2223.
32. Pietropaolo M. Pathogenesis of type 1 diabetes mellitus. UpToDate. Updated June 8, 2022. https://www.uptodate.com/contents/pathogenesis-of-type-1-diabetes-mellitus?search=diabetes%20pathophysiology&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2#H28
33. Robertson RP, Udler MS. Pathogenesis of type 2 diabetes mellitus. UpToDate. Updated Dec. 14, 2021. https://www.uptodate.com/contents/pathogenesis-of-type-2-diabetes-mellitus?search=diabetes%20pathophysiology&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H40
34. American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2022. Diabetes Care 2022;45(Suppl 1):S17-S38
35. Umpierrez GE, Davis GM, ElSayed NA, et al. Hyperglycemic crises in adults with diabetes: A consensus report. Diabetes Care. 2024;47:1257-1275.
36. Katz MA. Hyperglycemia-induced hyponatremia — calculation of expected serum sodium depression. N Engl J Med 1973;289:843-844.
37. Hillier TA, Abbott RD, Barrett EJ. Hyponatremia: Evaluating the correction factor for hyperglycemia. Am J Med 1999;106:399-403.
38. Cryer PE. Hypoglycemia in adults with diabetes mellitus. UpToDate. Updated April 19, 2021. https://www.uptodate.com/contents/hypoglycemia-in-adults-with-diabetes-mellitus?search=hypoglycemia&source=search_result&selectedTitle=3~150&usage_type=default&display_rank=3#H1
39. Heller SR, Peyrot M, Oates SK, Taylor AD. Hypoglycemia in patient with type2 diabetes treated with insulin: It can happen. BMJ Open Diabetes Res Care 2020;8:e001194.
40. Pitocco D, Di Leo M, Tartaglione L, et al. An approach to diabetic ketoacidosis in an emergency setting. Rev Recent Clin Trials 2020;15:278-288.
41. Gallo de Moraes A, Surani S. Effects of diabetic ketoacidosis in the respiratory system. World J Diabetes 2019;10:16-22.
42. Ghimire P, Dhamoon AS. Ketoacidosis. In: StatPearls [Internet]. StatPearls Publishing; Aug. 11, 2021.
43. Gosmanov AR, Gosmanova EO, Kitabchi AE, et al. Hyperglycemic crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. In: Endotext [Internet]. May 9, 2021.
44. Umpierrez GE. Hyperglycemic crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. In: Bonora E, Defronzo RA, eds. Diabetes Complications, Comorbidities and Related Disorders. Springer Nature Switzerland AG;2020:596-614.
45. Barski L, Eshkoli T, Branstaetter E, Jotkowitz A. Euglycemic diabetic ketoacidosis. Eur J Intern Med 2019;63:9-14.
46. Fanelli CG, Lucidi P, Bolli GB, Porcellati F. Hypoglycemia. In: Bonora E, Defronzo RA, eds. Diabetes Complications, Comorbidities and Related Disorders. Springer Nature Switzerland AG;2020:615-652.
47. Rewers A. Acute metabolic complications in diabetes. In: Cowie CC, Casagrande SS, Menke A, et al, eds. Diabetes in America. 3rd ed. National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
48. Long B, Willis GC, Lentz S, et al. Evaluation and management of the critically ill adult with diabetic ketoacidosis. J Emerg Med 2020;59:371-383.
49. Gosmanov AR, Gosmanova EO, Kitabchi AE, et al. Hyperglycemic crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. May 9, 2021. Endotext [Internet]. MDText.com, Inc.
50. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32:1335-1343.
51. Soleimanpour H, Taghizadieh A, Niafar M, et al. Predictive value of capnography for suspected diabetic ketoacidosis in the emergency department. West J Emerg Med 2013;14:590-594.
52. Chebl RB, Madden B, Belsky J, et al. Diagnostic value of end tidal capnography in patients with hyperglycemia in the emergency department. BMC Emerg Med 2016;16:7.
53. Baldrighi M, Sainaghi PP, Bellan M, et al. Hyperglycemic hyperosmolar state: A pragmatic approach to properly manage sodium derangements. Curr Diabetes Rev 2018;14:534-541.
54. Long B, Willis GC, Lentz S, et al. Diagnosis and management of the critically ill adult patient with hyperglycemic hyperosmolar state. J Emerg Med 2021;61:365-375.
55. Ramphul K, Joynauth J. An update on the incidence and the burden of diabetic ketoacidosis in the U.S. Diabetes Care 2020;43:e196-e197.
56. Dingle HE, Slovis C. Diabetic hyperglycemic emergencies: A systematic approach. Emerg Med Pract 2020;22:1-20.
57. Jalili M, Niroomand. Type 2 diabetes mellitus. In: Tintinalli JE, Ma OJ, Yealy DM, et al. Emergency Medicine: A Comprehensive Study Guide. 9th ed. McGraw-Hill Education;2020.
58. Eledrisi MS, Elzouki AN. Management of diabetic ketoacidosis in adults: A narrative review. Saudi J Med Med Sci 2020;8:165-173.
59. Muneer M, Akbar I. Acute metabolic emergencies in diabetes: DKA, HHS and EDKA. In: Islam MS, ed. Diabetes: From Research to Clinical Practice. Advances in Experimental Medicine and Biology, vol. 1307. Springer;2020. https://doi.org/10.1007/5584_2020_545
60. Pitocco D, Di Leo M, Tartaglione L, et al. An approach to diabetic ketoacidosis in an emergency setting. Rev Recent Clin Trials 2020;15:278-288.
61. Umpierrez GE. Hyperglycemic crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. In: Bonora E, DeFronzo RA, eds. Diabetes Complications, Comorbidities and Related Disorders. Springer Nature;2020:596-614.
62. Dhatariya KK. Defining and characterising diabetic ketoacidosis in adults. Diabetes Res Clin Pract 2019;155:107797.
63. Dhatariya K, Matfin G. Severe hyperglycemia, diabetic ketoacidosis, and hyperglycemic hyperosmolar state. In: Matfin G, ed. Endocrine and Metabolic Medical Emergencies: A Clinician’s Guide, 2nd ed. John Wiley & Sons Ltd.;2018:531-547.
64. Lizzo JM, Goyal A, Gupta V. Adult diabetic ketoacidosis. In: StatPearls [Internet]. Updated Nov. 20, 2021. StatPearls Publishing; 2022.
65. Aldhaeefi M, Aldardeer NF, Alkhani N, et al. Updates in the management of hyperglycemic crisis. Front Clin Diabetes Healthc 2022;Feb 9:820728.
66. Semler MW, Self WH, Wanderer JP, et al. SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018;378:829-839.
67. Farkas J. Diabetic ketoacidosis. Internet Book of Critical Care. Updated Aug. 6, 2021. https://emcrit.org/ibcc/dka/#fluid_administration
68. Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic acidosis–Ringer’s lactate versus normal saline: A randomized controlled trial. QJM 2012;105:337-343.
69. Mahler SA, Conrad SA, Wang H, Arnold TC. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med 2011;29:670-674.
70. Ramanan M, Attokaran A, Murray L, et al. SCOPE-DKA Collaborators and Queensland Critical Care Research Network (QCCRN). Sodium chloride or Plasmalyte-148 evaluation in severe diabetic ketoacidosis (SCOPE-DKA): A cluster, crossover, randomized, controlled trial. Intensive Care Med 2021;47:1248-1257.
71. Self WH, Evans CS, Jenkins CA, et al. Pragmatic Critical Care Research Group. Clinical effects of balanced crystalloids vs saline in adults with diabetic ketoacidosis: A subgroup analysis of cluster randomized clinical trials. JAMA Netw Open 2020;3:e2024596.
72. Dhatariya K; Joint British Diabetes Societies for Inpatient Care. The management of diabetic ketoacidosis in adults. (JBDS 02). Revised June 2021.
73. Haas NL, Gianchandani RY, Gunnerson KJ, et al. The two-bag method for treatment of diabetic ketoacidosis in adults. J Emerg Med 2018;54:593-599.
74. Munir I, Fargo R, Garrison R, et al. Comparison of a ‘two-bag system’ versus conventional treatment protocol (‘one-bag system’) in the management of diabetic ketoacidosis. BMJ Open Diabetes Res Care 2017;5:e000395.
75. So TY, Grunewalder E. Evaluation of the two-bag system for fluid management in pediatric patients with diabetic ketoacidosis. J Pediatr Pharmacol Ther 2009;14:100-105.
76. Velasco JP, Fogel J, Levine RL, Ciminera P, et al. Potential clinical benefits of a two-bag system for fluid management in pediatric intensive care unit patients with diabetic ketoacidosis. Pediatr Endocrinol Diabetes Metab 2017;23:6-13.
77. Veverka M, Marsh K, Norman S, et al. A pediatric diabetic ketoacidosis management protocol incorporating a two-bag intravenous fluid system decreases duration of intravenous insulin therapy. J Pediatr Pharmacol Ther 2016;21:512-517.
78. Cho N, Bushell T, Choi M, Moussavi K. Evaluation of the two-bag system in adult diabetic ketoacidosis patients. J Pharm Pract 2021;34:17-22.
79. Eledrisi MS, Beshyah SA, Malik RA. Management of diabetic ketoacidosis in special populations. Diabetes Res Clin Pract 2021;174:108744.
80. Plewa MC, Bryant M, King-Thiele R. Euglycemic diabetic ketoacidosis. In: StatPearls [Internet]. StatPearls Publishing; 2022.
81. Nasa P, Chaudhary S, Shrivastava PK, Singh A. Euglycemic diabetic ketoacidosis: A missed diagnosis. World J Diabetes 2021;12:514-523.
82. Cryer PE. Hypoglycemia in adults with diabetes mellitus. UpToDate. Updated April 19, 2021. https://www.uptodate.com/contents/hypoglycemia-in-adults-with-diabetes-mellitus?search=hypoglycemia&source=search_result&selectedTitle=3~150&usage_type=default&display_rank=3#H1
83. Haamid A, Christian E, Tataris K, et al. Prehospital intranasal glucagon for hypoglycemia. Prehos Emerg Care. 2023;27(3):356-359.
84. Lamos EM, Younk LM, Davis SN. Hypoglycemia. In: Matfin G, ed. Endocrine and Metabolic Medical Emergencies: A Clinician’s Guide, 2nd ed. John Wiley & Sons Ltd.;2018:506-530.
85. Fanelli CG, Lucidi P, Bolli GB, Porcellati F. Hypoglycemia. In: Bonora E, DeFronzo RA, eds. Diabetes Complications, Comorbidities and Related Disorders. Springer Nature;2020:615-652.
86. Muneer M. Hypoglycaemia. In: Islam MS, ed. Diabetes: From Research to Clinical Practice. Advances in Experimental Medicine and Biology. Springer;2020. https://doi.org/10.1007/5584_2020_534
87. Nakhleh A, Shehadeh N. Hypoglycemia in diabetes: An update on pathophysiology, treatment, and prevention. World J Diabetes 2021;12:2036-2049.
88. Chu J, Stolbach A. Sulfonylurea agent poisoning. UpToDate. Updated Aug. 12, 2020. https://www.uptodate.com/contents/sulfonylurea-agent-poisoning?search=sulfonylureas&source=search_result&selectedTitle=2~112&usage_type=default&display_rank=2#H1
89. Wolf RA, Haw JS, Paul S, et al. Hospital admissions for hyperglycemic emergencies in young adults at an inner-city hospital. Diabetes Res Clin Pract 2019;157:107869.
90. Rao P, Jiang S, Kipnis P, et al. Evaluation of outcomes following hospital-wide implementation of a subcutaneous insulin protocol for diabetic ketoacidosis. JAMA Netw Open 2022;5:e226417.
91. Vincent M, Nobécourt E. Treatment of diabetic ketoacidosis with subcutaneous insulin lispro: A review of the current evidence from clinical studies. Diabetes Metab 2013;39:299-305.
92. American Diabetes Association Professional Practice Committee. Introduction and methodology: Standards of care in diabetes — 2025. Diabetes Care. 2024;48:S1-S5. https://diabetesjournals.org/care/issue/48/Supplement_1