
Tracheostomy Patients in the Emergency Department
April 1, 2025
By Matthew Turner, MD, and Erica Bates, MD
EXECUTIVE SUMMARY
- Approximately 40% to 50% of tracheostomies will develop a complication, most of them minor.
- Emergency complications include accidental decannulation, obstruction, and hemorrhage.
- Most tracheostomy obstructions can be cleared with removal of attachments and the inner cannula, followed by suction.
- Bleeding within three weeks of tracheostomy placement should be urgently investigated for trachea-innominate artery fistula.
- For active severe bleeding, the tracheostomy cuff should be hyperinflated and the tube adjusted to provide pressure against the anterior tracheal wall.
- Bougies or other stiff introducers should not be used for tracheostomy replacement because of the risk of creating a false passage.
- With acute respiratory failure, if ventilation is not possible through the tracheostomy tube, it should be removed and bag valve mask ventilation should be done with occlusion of the tracheal stoma.
Introduction
Tracheostomy has become an increasingly common procedure in recent years and is now performed on approximately 8% to 13% of intensive care unit (ICU) patients. It may be done for long-term mechanical ventilation, as a long-term solution to threatened upper airway obstruction, or for a number of other reasons.1 (See Table 1.) Tracheostomy can be performed through a number of different methods, including percutaneous tracheostomy performed in the ICU, through surgical tracheostomy performed in the operating room, or through a hybrid approach.1 More than 100,000 percutaneous tracheostomies are performed annually in the United States.2 The only absolute contraindication for tracheotomy is cellulitis of the anterior neck.3
Table 1. Common Indications for Tracheostomy Placement | |
Category | Examples |
Upper airway obstruction |
|
Need for prolonged mechanical ventilation |
|
Inability to intubate |
|
Airway protection |
|
Adjunct |
|
Despite the relative safety of the procedure, 1% of tracheostomy patients will experience a catastrophic complication, with up to half of these patients dying. Given this, it is important that emergency physicians (EPs) be aware of the potential complications and unique challenges that tracheostomy patients may face.4
Tracheostomy Structure
While debate over the correct terminology is ongoing, tracheotomy refers to the procedure, while tracheostomy refers to the airway created by the procedure.5 It is important for an EP to understand the difference between a tracheostomy and a laryngectomy, which have very different implications for emergency airway management. During a tracheostomy, a stoma is created in the trachea to form an airway in the neck, but anatomical connection is preserved between the mouth and lungs. Depending on the indication for the tracheostomy and the patient’s anatomy, it may be possible to oxygenate via supplemental oxygen applied to the face or even to orotracheally intubate a patient with a tracheostomy should the stoma become unusable.
In contrast, during a total laryngectomy, the connection between the mouth and lower airway/lungs is completely removed, and the only remaining passage for air to enter the lungs is through the surgical airway created in the neck. Laryngectomy patients cannot be oxygenated or intubated through the nose/mouth, and airway emergencies require a different approach focused solely on accessing their remaining surgical airway. This article will focus on patients with the more common procedure, tracheotomy with creation of a tracheostomy.
Tracheostomy tubes may be cuffed or uncuffed and include both an outer and inner cannula.6 (See Figures 1 and 2.) The tracheostomy tube consists of a shaft, typically ranging from 60 mm to 90 mm, attached to a flange that is meant to be secured against the patient’s neck. When properly fitted, flanges have less risk of causing discomfort, retained moisture, and skin breakdown.7
Figure 1. Metal Tracheostomy Tube — Jackson Design |
1A. (top) Disassembled into three pieces, outer cannula with flange, obturator, and inner cannula 1B. (bottom) Assembled for insertion with obturator inserted through inner cannula |
 |
Image courtesy of J. Stephan Stapczynski, MD. |
Figure 2. Plastic Tracheostomy Tube — Shiley Design with Inflatable Cuff |
2A. (top) Disassembled into three pieces, outer cannula with flange and inflatable cuff, obturator, and inner cannula 2B. (bottom) Assembled for insertion with obturator inserted through inner cannula |
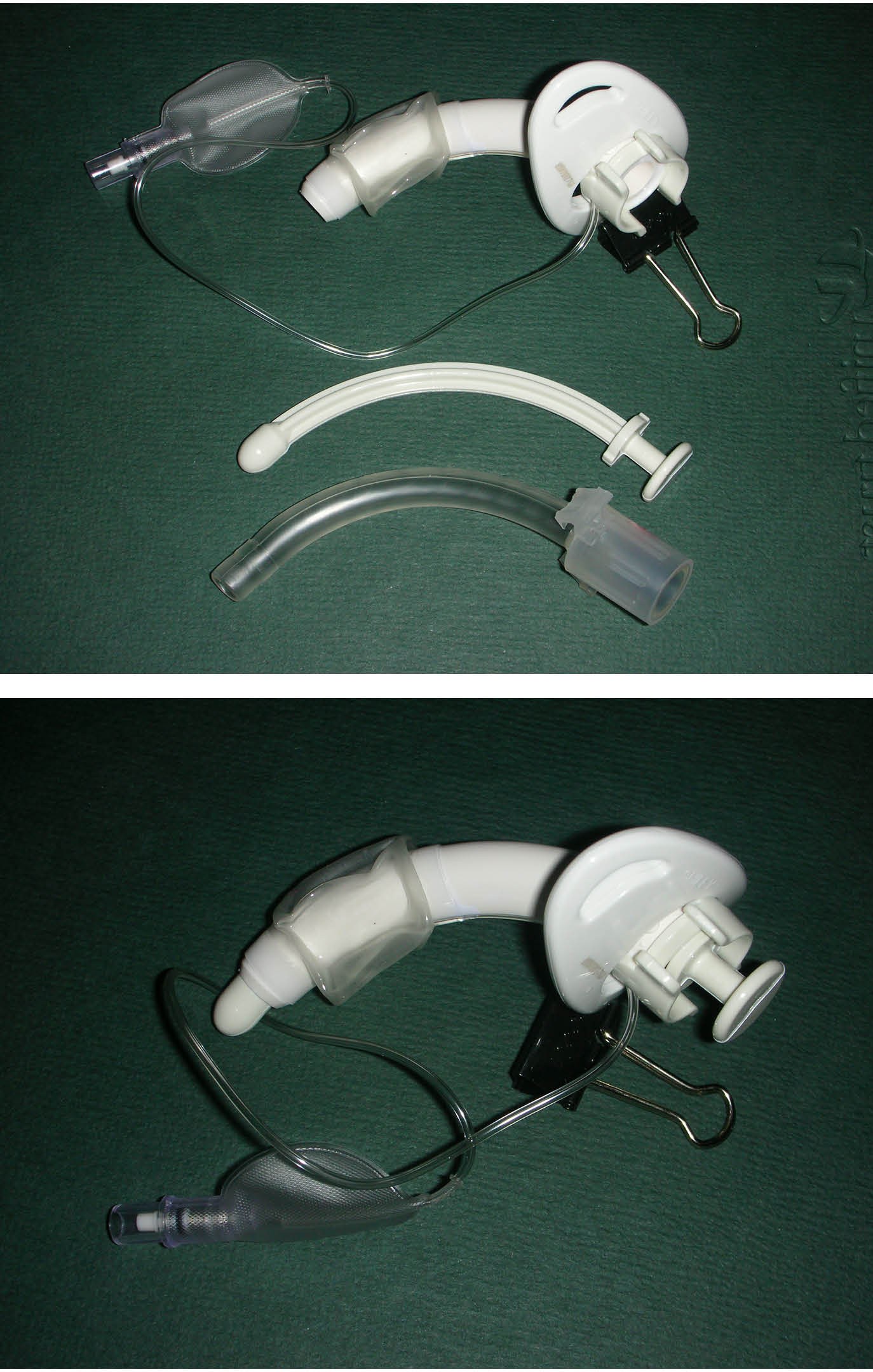 |
Image courtesy of J. Stephan Stapczynski, MD. |
The majority of tracheostomy tubes will have a 15-mm connector that allows ventilatory equipment, such as a bag valve mask (BVM) or mechanical ventilator, to be directly connected to the tube. A removable inner cannula that fits inside the main shaft also may be present. The inner cannula can be removed easily or replaced to clean secretions without changing the entire tracheostomy tube, but it also reduces the inner diameter of the tube.7 Some tubes also may be fenestrated to help with vocalization by allowing air to flow to the larynx, which subsequently makes them more vulnerable to blockage by sputum or granulation tissue.7 Other attachments, such as speaking valves and external humidifiers, also may be present.8
A cuffed tracheostomy tube may be used to allow for assisted ventilation or to reduce the risk of pulmonary aspiration. Along with these advantages, inflating a cuffed tube comes with its own risks. Cuff pressure typically should be maintained at 20 mmHg. Cuff pressures above 30 mmHg can compress mucosal capillaries and inhibit blood flow; pressures above 40 mmHg are equal to the perfusion pressure of the tracheal mucosa and increase the risk of ulceration, bleeding, stenosis, and fistula. Pressures above 50 mmHg can cause complete occlusion of blood flow. Cuff volumes usually should be maintained at approximately 6 mL to 8 mL, since cuffs inflated to more than 10 mL also raise the risk of injury to the trachea.9
Of note, tracheostomy devices are not confined to traditional tubes with an inner and outer cannula. In addition to tracheostomy tubes, patients also may have a stoma maintenance device — a small uncuffed tube or stent that is meant to maintain the patency of the stoma but does not have a tube that projects into the tracheal lumen.6 A tracheostomy button is even simpler, consisting of a single hollow cannula from the skin to the anterior wall of the trachea that allows for respiration.6 In rare cases of severe tracheal stenosis, patients may have a T-tube as a bridge to definitive treatment. T-tubes consist of a main vertical tube that serves as a stent within the trachea, and a horizontal limb that protrudes through the stoma and acts as a tracheostomy tube.6 T-tubes are particularly difficult to manage in emergency settings and will be discussed further in the airway management section.
Emergent Complications
Approximately 40% to 50% of tracheostomies will develop some sort of complication, the vast majority of which are relatively minor. However, up to 1% of tracheostomy patients will experience a catastrophic, potentially life-threatening complication.4 Complications can be considered emergent, acute/subacute, or chronic. (See Table 2.)
Table 2. Emergent, Acute/Subacute, and Chronic Complications of Tracheostomy |
Emergent Complications
Acute/Subacute Complications
Chronic Complications
|
Tracheostomy Decannulation
Accidental tracheostomy decannulation (ATD) is an emergent, potentially life-threatening complication, particularly within the first week of placement.11 While decannulation is a spectrum — ranging from full displacement of the tracheostomy tube to partial displacement — it always should be treated as an emergent threat.4 Reported ATD rates in the early postoperative period range from 0.7% to 20%.12 It may occur for a number of reasons, such as a reduction in neck circumference because of weight loss in injured, septic, or postpartum patients; accidental pulling on the tracheostomy tube; or drowsiness and restlessness associated with the immediate postoperative period.13 Pediatric patients may experience ATD as a result of pulling on the tubes, failure to properly secure the tracheostomy, or even through severe coughing.14
In cases of ATD, it is essential that emergency department (ED) physicians determine the timing of when the tracheostomy was placed and when the tracheostomy became decannulated. Because of the lack of mature stoma formation, any decannulation within seven days of tracheostomy placement should be treated as an emergent airway. However, any replacement of the cannula should be done only through fiberoptic endoscopy during this time frame, because of the risk of causing a false passage through the soft tissue of the neck.4 If fiberoptic endoscopy is not available, patients may require oral intubation for proper airway management, with the cuff inflated distal to the stoma.2,4 Any blind placement of an endotracheal tube through the stoma in the acute phase should be strictly avoided because of the high risk of creating a false passage, particularly in obese patients.2
Even in cases where the tracheostomy is more than seven days old, decannulation still can be an emergent complication. Even mature stoma may narrow within hours after ATD.4 Other decannulation complications also may include uncontrolled secretions, severe glottic stenosis, and a high risk of aspiration.15,16 If the tracheostomy is older than seven days, a new tracheostomy tube may be carefully inserted, then confirmed via fiberoptic visualization. If the physician encounters resistance while inserting the new tube, they should reattempt with a smaller tracheostomy tube or use an analogous-sized endotracheal tube if no tracheostomy tubes are available. Patients still may require BVM oxygenation and even oral intubation if they are having difficulty oxygenating or ventilating during this process.4
Tracheostomy Obstruction
Obstruction of the tracheostomy tube is another potentially life-threatening complication.4 It may result from a number of factors, including mucus plugging, clotted blood, impingement of the tracheostomy tube against the posterior tracheal wall, granulation tissue, or displacement of the tube into a false lumen.4
While potentially life-threatening, the management of tracheostomy obstructions is relatively straightforward.4 Any potentially obstructing attachments, such as speaking valves, a decannulation cap, bandages, or humidifying devices, should be removed, as well as the inner cannula, if present. Afterward, suctioning should be performed through the tracheostomy tube. This will relieve the majority of obstructions. However, if the suction cannot be fully advanced through the tracheostomy tube, this indicates that a complete obstruction is present.4 In these cases, no bougies or rigid tubes should be introduced into the tracheostomy tube because of the risk of forming a false passage.8 The distal cuff then should be deflated. If there still is blockage of the tracheostomy tube afterward, the tube should be removed, since a non-functioning tracheostomy tube will do more harm than good for the patient.8
With the tube removed, ventilation may be attempted via a BVM. Ventilation can be attempted with either a pediatric face mask or a laryngeal mask airway placed over the stoma.4 If the BVM is used over the patient’s mouth, the tracheal stoma should be occluded. If there still is concern for an occluded airway, the patient may be either intubated through the oropharynx, with the endotracheal tube advanced and inflated beyond the stoma, or intubated through the stoma if the tracheostomy is more than seven days old.4
Hemorrhage
Bleeding is a common complication from tracheostomy placement, with an incidence of approximately 5.7%.17 The majority of cases are superficial and will be discussed later. However, development of a trachea-innominate fistula (TIF), while rare with an incidence of 0.7%, is extremely deadly, with a mortality of more than 90%.4 The innominate artery crosses over the anterior trachea from the left inferolateral to the high right anterolateral position. While other vessels, such as the common carotid artery and inferior thyroid artery, may be involved, this specific anatomy makes the innominate artery especially prone to developing a fistula into the trachea after a tracheostomy.
TIFs develop due to the tracheostomy tube eroding into the innominate artery. Elevated cuff pressures, repetitive head movements, a lower tracheostomy insertion, and other mechanisms that increase anterior pressure on the tracheostomy all increase the risk.17 Other risk factors include a low-placed tracheostomy tube, an anomalous high-riding innominate airway, and infection of the tracheostomy site.4 Patients with chest deformities, which may result in increased contact between the innominate artery and the tracheal wall, also appear to be at increased risk.18
The majority of cases occur within three weeks of tracheostomy placement, with a peak incidence between the first and second weeks, but they may occur at any time. Approximately 50% of TIF bleeds display a sentinel event, which can present as hemoptysis or blood seen with routine suctioning.4 Given the extremely high mortality associated with this condition, any evidence of tracheostomy-associated hemorrhage, especially within the first several weeks following tracheostomy placement, should be considered TIF until proven otherwise.4 Prompt diagnosis with either bronchoscopy, computed tomography (CT) angiogram of the neck, or local exploration should be performed, with appropriate input from surgical consultants.4
In cases where active bleeding from a TIF is suspected, several methods to temporize the bleed may be employed. If possible, the tracheostomy tube should be hyperinflated and adjusted so that it provides pressure against the site of the hemorrhage.4,19 An endotracheal tube also may be hyperinflated in a similar maneuver.19 External compression at the sternal notch also may be attempted.4 If the hemorrhage continues, physicians may employ the Utley Maneuver, in which a gloved finger is inserted into the stoma to perform direct digital compression of the TIF against the posterior sternum.19 Ultimately, these measures all are temporary; surgical intervention, through either median sternotomy or endovascular intervention, remains the standard of care.19
Acute and Subacute Complications
Bleeding
As discussed earlier, bleeding is the most common complication of tracheostomy and often is seen in the acute postoperative phase, or in the subacute phase (here defined as within seven days of the procedure). Perioperative bleeding may occur because of damage to the local vasculature, and often can be minimized by preoperative ultrasound to identify any significant vessels in the field of interest.17 Subacute bleeding within the first 48 hours after the procedure usually is minor due to superficial veins.2
Cases of mild bleeding around the stoma, where TIF is not suspected, may be treated with local pressure and packing with gauze soaked in epinephrine or tranexamic acid.2 Topical agents, such as silver nitrate or Surgicel, also may be used.20 While acute and subacute bleeds typically are benign, in patients with respiratory failure and a low pulmonary reserve, even 150 mL to 200 mL of blood can lead to hypoxia and ventilatory failure.2 Any bleeding 48 hours after tracheostomy placement should be assessed aggressively, since up to 50% of these bleeds are caused by TIF.10 Emergency physicians should have a low threshold for consultation in cases of tracheostomy bleeding.
Pneumothorax
Pneumothorax secondary to tracheostomy has an incidence reported from 0.8% to as high as 17%.2,10 Postoperative chest radiography often is ordered routinely after tracheostomy placement, but this should not be taken for granted — recent studies have concluded that avoiding routine chest radiography after tracheostomy may be helpful in reducing costs without altering overall management.10 Pneumothorax after tracheostomy placement is associated with chronic obstructive pulmonary disease (COPD), obesity, spinal abnormalities, and operator inexperience. Because of the risk of death from pneumothorax, ED physicians should be aware of this complication in the acute phase.2
Tracheal Damage
Percutaneous placement of tracheostomy may cause damage to the posterior tracheal wall by the guidewire, possibly injuring the esophagus as well.10 The majority of tracheal wall tears are small and benign and self-resolve with conservative treatment.2 Generally, clinically stable patients with small tracheal tears less than 2 cm, with minimal subcutaneous emphysema and pneumomediastinum, may be treated non-surgically.2 Larger tears may lead to bleeding into the airway, pneumomediastinum, and subcutaneous emphysema, as shown in Figure 3. These cases should be treated as a surgical emergency.17 Patients who are older adults, short in stature, or have COPD are more vulnerable to injury to the posterior tracheal wall.10
Figure 3. Subcutaneous Emphysema Due to Malpositioned Tracheostomy Tube |
Chest X-ray displaying a massive subcutaneous emphysema from a malpositioned tracheostomy tube that developed while the patient was on the ventilator. The tracheostomy tube was replaced by an endotracheal tube. |
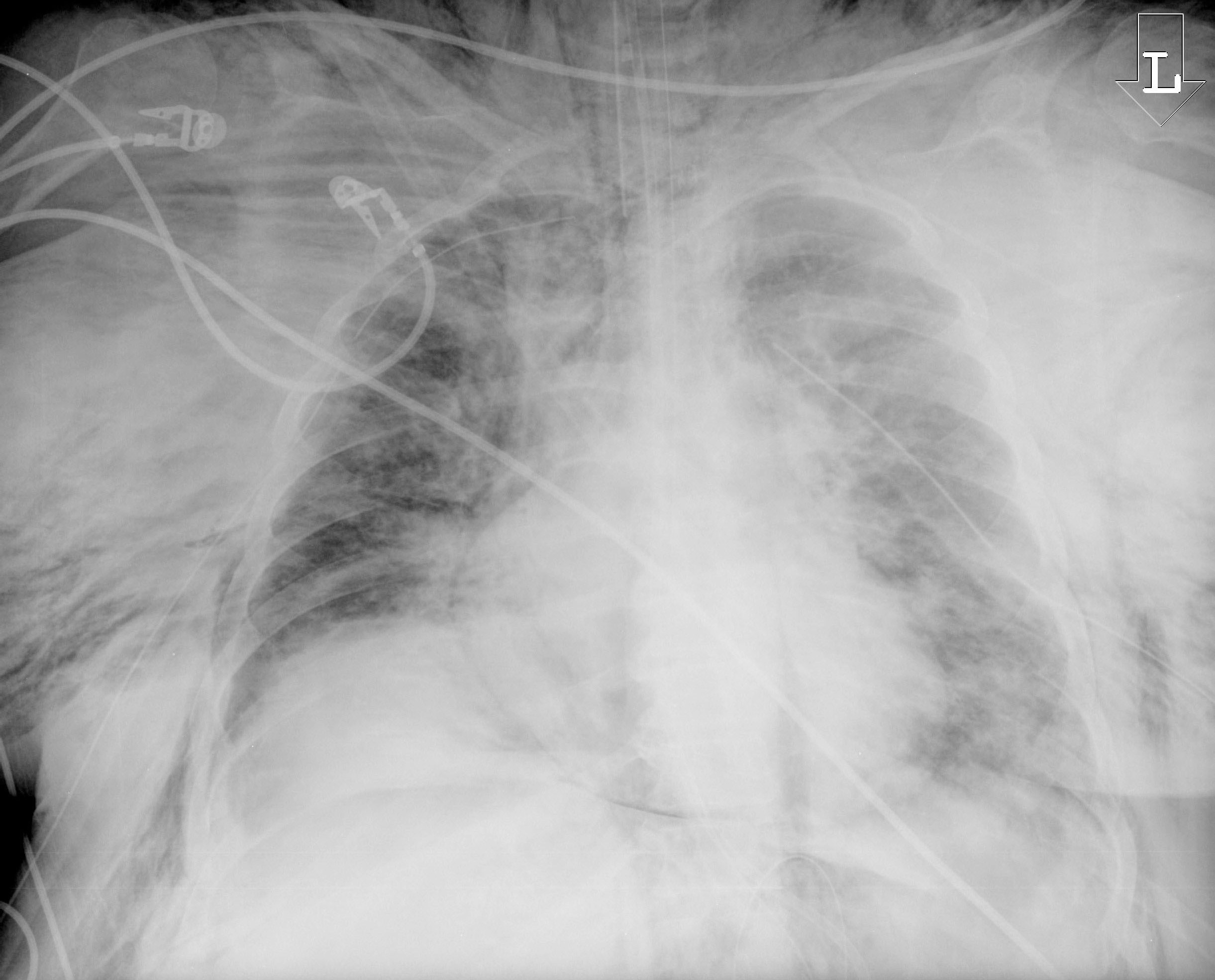 |
Image courtesy of J. Stephan Stapczynski, MD. |
Infections and Aspiration
Because the site of the tracheostomy is considered to be a “clean-contaminated location,” it is common for patients to develop cellulitis and tracheitis in the early postoperative course.10 While these infections typically are minor, more severe complications, including sternal wound infections and mediastinitis, may result.4,21 A 2017 meta-analysis of 13 studies found that patients undergoing cardiac surgery after tracheostomy had an incidence of sternal wound infection of approximately 7%.21 A similar incidence was found in early (< 14 days after tracheostomy) and late (≥ 14 days) post-tracheostomy patients, suggesting that tracheostomy may increase the risk of postoperative infection regardless of the timing.21 Infection is a long-term risk as well; risk factors include male patients, patients with diabetes, and the use of cuffed tubes.22
Because of the high incidence of dysphagia, ranging from 11% to 93%, aspiration also poses a significant risk in this patient population.23 The mechanics of how the tracheostomy tube affects swallowing remain highly debated in the literature, but it appears that the presence of the tube results in desensitization, uncoordinated glottic closure, and atrophy of the muscles of the throat, which ultimately impairs the swallowing reflex.24,25 If possible, these patients should be assessed by speech and language therapy (SLT) to minimize the risk of an aspiration event.24 Medically complex patients, such as those with deconditioning from their critical illness; prior risk factors for dysphagia; recent retropharyngeal abscess or hematoma; recent oral, pharyngeal, or laryngeal surgery; or recent cancer of the neck, are significantly more likely to develop aspiration events.26
Excessive secretions also increase the risk of an aspiration event and should be managed through regular suctioning of the tracheostomy tube. In more extreme cases of hypersecretion, medications, such as sublingual atropine (one to two drops twice or three times per day), glycopyrrolate, or even botulinum toxin injections into the salivary glands, may be used.25
The site of the tracheostomy stoma is another potential source of infection, with an incidence of approximately 5% to 6%, typically more than 24 hours after placement. These infections typically are limited and may be treated with local antibiotics, frequent dressing and tube changes, and humidification of inspired air.10,20 The stoma always should be kept clean and dry, with regular cleanings via sterile water or normal saline. If copious secretions are present, patients may require frequent dressing changes and barrier creams.20 Regular cleaning and inspection should be performed to minimize the risk of skin breakdown around the site.
In some cases, patients may require different dressings, such as gauze drain sponges for mild secretions, polyurethane foam for copious secretions, hydrocolloid for skin breakdown, or specialized silver-containing products for local infections of the stoma.20 In severe cases, stomal infection may worsen to necrotizing tracheal infection. If this is suspected, patients should be given broad-spectrum systemic intravenous (IV) antibiotics, have a secure airway placed via orotracheal intubation, have their tracheostomy tube removed, and undergo emergent surgical intervention.20
Mediastinitis may result from an infected stoma, since the infection travels through the fascial planes of the neck down into the mediastinum. This infection is associated with a high mortality and should be treated aggressively.10 Other potential infections for the EP to consider include tracheitis, an elevated risk of pneumonia, and even sternoclavicular osteomyelitis.10
Tracheoesophageal Fistula
Approximately 0.5% to 1% of patients will develop a tracheoesophageal fistula (TEF) after tracheostomy. This complication is associated with excess cuff pressure, prolonged intubation, use of a nasogastric (NG) tube, type 1 diabetes mellitus, steroid use, a history of gastroesophageal reflux disease (GERD), anemia, and sepsis.4,27 An overinflated cuff can cause prolonged pressure on the posterior wall of the trachea, leading to ischemia, necrosis, breakdown, and, finally, fistula formation.4 Patients with TEF can present with tracheal air leaks, increased aspiration events, abdominal distension, respiratory distress, or significant secretions.
Tracheoesophageal fistulas are diagnosed through either bronchoscopy or esophagram, and a high degree of suspicion may be required for the EP to consider this potential internal cause of ongoing aspiration.4 The majority of TEFs will not spontaneously close and will require surgical treatment.28 In the ED, patients should have oral intake discontinued, should have the head of their bed elevated to 45 degrees, and should undergo tracheal suctioning. If a nasogastric tube is present, it should be removed.4 Specialist consultation, with a surgeon or potentially a gastroenterologist at some institutions, will be required to address the fistula.
Late Complications (More than Seven Days)
Tracheal Stenosis
Post-tracheostomy tracheal stenosis (PTTS) occurs because of the abnormal narrowing of the tracheal lumen after tracheostomy, either due to fibrosis or granulation tissue. While it is one of the most common complications of tracheostomy, only 3% to 12% of patients will develop clinically significant stenosis.2 A recent 2021 study estimated an incidence of tracheal stensosis of 8.8% and found that only severe cases of stenosis were symptomatic.29 Generally, patients will develop early symptoms, such as cough and difficulty in clearing secretions, when the tracheal lumen is less than 50% of its original diameter.2 As the lumen further narrows, patients may display exertional dyspnea, stridor, and even respiratory failure because of reduction of the upper airway diameter.2 Post-tracheostomy tracheal stenosis can occur at the level of the stoma, the location of the cuff, or even at the distal tip of the tracheostomy.2 A retrospective study of 99 PTTS patients estimated a median time from tracheostomy injury to stenosis detection of approximately four months.30
Risk factors for PTTS include repeated surgical tracheostomies, steroid use, advanced age, severe GERD, autoimmune diseases, obstructive sleep apnea, and a history of radiation to the throat.2,29 These patients will require bronchoscopic intervention through the use of stents, laser cauterization, or ballooning.30
Tracheomalacia
Weakening of the tracheal wall from ischemic injury leading to necrosis and destruction of the supporting cartilage can lead to the excessive expiratory collapse of the trachea, a condition known as tracheomalacia. Patients with tracheomalacia will develop expiratory flow limitation and air trapping, and will not be able to properly clear their secretions. Post-tracheostomy patients with tracheomalacia often present with cough and worsening dyspnea.2
Of note, patients with a tracheostomy tube already in place also can develop tracheomalacia. In this case, it is recommended that a longer tracheostomy tube be placed to bypass the area of weakened tracheal wall.2 In the acute setting, patients presenting to the ED with concerns for tracheomalacia should be administered noninvasive positive pressure ventilation.2 Continuous positive airway pressure (CPAP) appears to be effective in tracheomalacia because of its ability to stent the airway open.31 Ultimately, these patients will require bronchoscopy, with the possibility of tracheoplasty, tracheal resection, or stent placement.2
Tracheal Granuloma
Tracheal granulomas have an incidence of approximately 7% in post-tracheostomy patients.3 Formed by the friction between the tracheostomy tube and inner wall of the trachea, granulomas develop from the inflammatory response that induces cell proliferation at the site.3 Granulomas pose several risks to the patient. If large enough, they can obstruct the airway, increasing the risk of pneumonia and death. Granulomas also are fragile and highly vascularized, with a risk of significant bleeding.3 GERD, elevated age, smoking history, diabetes, prolonged intubation, repeated tracheotomies, and a history of pulmonary infections all are associated with an increased risk of developing tracheal granulomas.3 Approximately 50% of tracheal granulomas develop within 52 days of removal of the tracheostomy tube.3
Imaging via CT and magnetic resonance imaging (MRI) of the lung and neck often is limited in detecting tracheal granulomas, which may be difficult to differentiate between secretions and normal tracheal folds.3 Definitive diagnosis should be made via bronchoscopy, with surgical intervention as indicated.3
Prolonged Cuff Inflation
Ultimately, deflation of the tracheostomy tube depends on several factors, including the patient’s cough strength, bulbar function, and ability to spontaneously swallow saliva. Some patients may require a prolonged period with their tracheostomy tube fully inflated.32 However, this puts the patient at risk for increased complications.
Prolonged cuff inflation of the tracheostomy tube may lead to a number of chronic complications in a tracheostomy patient, including reduced cough strength, an increased risk of aspiration, stasis of secretions, and, as a result of impaired communication, increased frustration, anxiety, and other negative effects on the patient’s well-being and sense of identity.25,32
In addition to this, the patient’s tracheostomy cuff should be maintained at 20 mmHg to 25 mmHg. Overinflation of the cuff increases the risk of ischemia, then ulceration, necrosis, TIF, tracheal stenosis, and tracheomalacia. However, underinflation of the cuff below 15 mmHg raises the risk of aspiration pneumonia.20 If possible, the patient’s cuff pressure should be re-assessed every time the tracheostomy tube is adjusted.20
Tracheocutaneous Fistula
The majority of stomas close within six weeks of tracheostomy decannulation. However, if the stoma persists after three to six months, the patient may have developed a fistula due to persistent epithelization of the tract. Patients may present with local infection and skin irritation, persistently draining secretions, aspiration causing recurrent pneumonias, and a weak cough. Patients with a prolonged tracheostomy course, advanced age, malnutrition, and steroid use are at a higher risk of developing a cutaneous fistula. These tracts may be managed via cauterization, excision, or surgical repair.4 Pediatric patients are at particularly high risk of developing these fistulas; one study found an incidence of 37.8% in the pediatric population.33
Pediatric Patients
In the United States, approximately 4,800 tracheostomies are performed each year in pediatric patients.14 While emergent tracheotomies due to infections by organisms such as Haemophilus influenzae and Corynebacterium diphtheria have significantly decreased, the overall improvement of pediatric critical care has led to an increasing number of children with prolonged ventilation requiring tracheostomies.33 Up to 20% of these patients will experience emergent complications that require immediate assessment and intervention.14
The majority of pediatric patients who receive a tracheostomy are younger than 1 year of age.34 Although the safety of the procedure has significantly improved in recent decades, pediatric patients with tracheostomy have higher rates of morbidity and mortality than adult patients because of several factors, including premature birth, low birth weights, and more serious underlying diseases.35
Physicians should be aware of the “red flags” associated with tracheostomies in children that warrant emergent workup. (See Table 3.)
Table 3. Pediatric Tracheostomy Red Flags | |
Airway Red Flags
| Breathing Red Flags
|
Tracheostomy-Specific Red Flags
| General Red Flags Any physiological changes can be due to an airway problem. Specifically, changes in:
|
Reprinted with permission from: Doherty C, Neal R, English C, et al. Multidisciplinary guidelines for the management of paediatric tracheostomy emergencies. Anaesthesia. 2018;73:1400-1417. | |
In the immediate postoperative phase, pediatric patients have a higher incidence of pneumothorax than adults, likely because of anatomical differences.10 Accidental decannulation and obstruction are the two primary causes of tracheostomy-related emergencies in pediatric patients, with a mortality rate of 4% to 6%.14 Unattended, highly mobile children such as toddlers are more likely to experience accidental decannulation from tugging on the tracheostomy tubes.14 Likewise, pediatric patients are more likely to experience obstruction of their tracheostomy tubes because of mucus obstruction and smaller tube sizes.14 In addition to this, re-insertion of tracheostomy tubes also is more likely to result in the formation of false passages because of the shorter neck anatomy found in pediatric patients.14
Granulomas and infections — whether of the stoma, the trachea, surrounding cellulitis, or pneumonia — also are common complications in pediatric patients.35 Preterm infants appear to be the most likely to develop these complications, with more than 60% developing complications such as skin breakdown, tracheitis, and granulations.36
Tracheostomy-Associated Respiratory Infections
More than 20,000 children with tracheostomies are hospitalized each year, most commonly due to TRacheostomy-Associated respiratory INfections (TRAIN). While the definition of TRAIN is not fully standardized, it most often includes bacterial pneumonia and tracheitis.37 Children with tracheostomies frequently are chronically colonized with bacteria such as Pseudomonas aeruginosa and Staphylococcus aureus, making the optimal treatment of TRAINs difficult. Identifying optimal antimicrobial treatment often is challenging based on prior respiratory and sputum cultures; empiric antibiotics based on past cultures only provide proper antimicrobial coverage in 56% of patients.37
Unfortunately, there are no consensus guidelines for diagnosis or treatment of suspected TRAIN in pediatric patients with tracheostomy.38 The majority of these patients will receive chest radiographs and laboratory tests, such as complete blood count (CBC), blood cultures, and respiratory cultures, on initial admission.38 Treatment often includes empiric antibiotics that target Pseudomonas, but the efficacy of this appears limited. One retrospective study of 4,137 pediatric patients found that patients who received anti-pseudomonal coverage had a longer length of stay, possibly because of antibiotic resistance, limited enteral alternatives, or other confounders.38
Airway Management
Patients with tracheotomies present a difficult airway challenge. Hospitalized patients benefit from a sign placed on their bed providing information about their difficult airway, although this may be challenging to implement in an ED.8
When there is concern about a potentially compromised airway in these patients, physicians should follow a simple and stepwise approach. First, the patient’s airway should be assessed. Given that tracheostomy patients have two airways — the native upper airway and the tracheostomy tube — both of these should be assessed by looking, listening, and feeling at both sites for 10 seconds. If available, waveform capnography should be used on both airways to provide further confirmation of breathing.8 Patients should be optimally positioned for airway support, including chin lift and jaw thrusting in pediatric patients, or a pillow or rolled towel beneath the shoulders of a child younger than 2 years of age.39 Signs of respiratory distress, including stridor, accessory muscle use, and retractions, should be assessed carefully.39
If the patient is spontaneously breathing, they should have high-flow oxygen applied to both airways. In limited resource settings, this may require an oxygen cylinder to be brought into the room to provide a second oxygen supply.8 If there is no spontaneous breathing, the patient’s pulse should be assessed, and cardiopulmonary resuscitation (CPR) should be initiated if necessary.8
Because of the structure of the tracheostomy tube, several steps should be performed to assess patency of this airway. If an inner tube is present, it should be removed from the tracheostomy tube, because of the risk of obstruction. Other devices, including humidifying (HME or heat moisture exchange) devices such as the Swedish or artificial nose or speaking valves, also should be removed, because of their potential to become blocked from secretions.8 Once these supplemental devices have been removed, a suction catheter should be passed through the tracheostomy tube to ensure that the airway is patent. The suction catheter should be able to pass easily through the entirety of the tube and into the trachea. If it is unable to pass easily, this indicates that an obstruction is present.8 Bougies or other stiff introducers should not be placed through the tracheostomy tube because of the risk of creating a false passage.6
If an obstruction is present, the distal cuff of the tracheostomy tube should be deflated if possible to improve airflow. If there is no subsequent improvement, the tube may be completely obstructed. In these cases, if the patient is unable to breathe around the tracheostomy tube, the tube should be removed, since at this point its continued presence will do more harm than good for the patient.8 Afterward, both airways should be reassessed.8 In cases where the tracheostomy tube is not patent or has become completely displaced from the neck, replacement or reinsertion of the tracheostomy tube may be attempted.39 The initial attempt may be done with the same sized tube; if a subsequent attempt is needed, it should be done with a tube that is one-half size smaller.39 In pediatric patients, a soft suction catheter may be done as a third attempt to replace the tracheostomy tube, used in the Seldinger technique with the tracheostomy tube advanced over the soft suction catheter.39
If the patient does not improve, emergency oxygenation should commence. Ventilation through the native upper airway should be done with occlusion of the tracheal stoma to maximize effective oxygenation. The tracheal stoma also may be ventilated through either a pediatric face mask or a laryngeal mask airway applied to the skin, with the upper airway occluded by closing the nose and mouth.8 However, vigorous ventilation through the tracheostomy tube should be minimized — excessive positive pressure can cause pretracheal and mediastinal emphysema.6
If these attempts do not work, a definitive airway will have to be obtained. There are several choices available, depending on clinician expertise and available equipment.8 The patient’s history also should be considered. For example, a patient with a tracheostomy placed for severe stenosis of proximal trachea, or for a total laryngectomy, will not be able to be intubated orally. Similarly, if patients have had a previous prolonged course of intubation or tracheostomy placement, they are more likely to have significant tracheal inflammation, with granulomatous tissue prone to bleeding and obstruction.6
The timing of the tracheostomy also is extremely important. Any tracheostomy placed within seven days may not have a mature stoma, raising the dangerous risk of false passage, hemorrhage, subcutaneous emphysema, pneumomediastinum, and infection with any exchange of tracheostomy tubes.6 If possible, oral intubation may be performed, provided that the endotracheal tube is advanced beyond the level of the stoma. In cases of a long-established tracheostomy or a difficult upper airway, intubation may be attempted through the stoma, through either insertion of a smaller tracheostomy tube or through use of a bougie or fiberoptic scope to allow an endotracheal tube to be secured into the trachea.8 It should be noted that re-insertion of a tracheostomy tube in morbidly obese patients, patients with a short and thick neck, and in cases of an immature stoma may be extremely challenging.6
In the most extreme cases, where the patient cannot be oxygenated by either the native airway or the tracheostomy site, patients may require extreme measures, including cricothyroidotomy.39
T-Tube Patients
Patients who have a T-tube in place, rather than a more common tracheostomy tube, have particularly difficult airways because of the small size of the horizontal limb of the T-tube, the risk of leakage from the upright vertical limb within the trachea, and the small diameter of the T-tube itself.6
Airway management of the T-tube patient should consider the risk of leakage from the proximal end of the vertical limb. Given this, several different methods can be employed for airway management in these patients. First, the external limb protruding through the tracheostomy stoma can be plugged, and a supraglottic device can be used to provide airway management. Second, a 4.5-5.0 cuffed tracheal tube could be inserted into the horizontal limb of the T-tube and secured, while a clamped supraglottic device is placed to occlude the proximal aspect of the vertical T-tube. As a last resort, the T-tube could be removed entirely by pulling the horizontal limb through the stoma and removing the entire tube, with a small cuffed tracheal tube inserted through the stoma to achieve ventilation; however, this carries with it the risk of collapsing the trachea the T-tube was meant to support.6
Conclusion
Tracheostomies are prone to complications. Most complications are minor and can be readily treated. Serious and life-threatening complications require prompt recognition and expeditious management.
Matthew Turner, MD, is Emergency Medicine Resident, Penn State Health, Hershey, PA. Erica Bates, MD, is Assistant Professor, Emergency Medicine, Penn State Health, Hershey, PA.
References
1. McGrath BA, Brenner MJ, Warrillow SJ, et al. Tracheostomy in the COVID-19 era: Global and multidisciplinary guidance. Lancet Respir Med. 2020;8(7):717-725.
2. Zouk AN, Batra H. Managing complications of percutaneous tracheostomy and gastrostomy. J Thorac Dis. 2021;13(8):5314.
3. Li W, Hu Y, Hu Y, et al. Relative factors analysis of the occurrence and location of intratracheal granuloma following tracheotomy. Intern J Gen Med. 2024:6355-6365.
4. Bontempo LJ, Manning SL. Tracheostomy emergencies. Emerg Med Clin North Am. 2019;37(1):109-119.
5. Mitchell RB, Hussey HM, Setzen G, et al. Clinical consensus statement: Tracheostomy care. Otolaryngol Head Neck Surg. 2013;148(1):6-20.
6. Rosero EB, Corbett J, Mau T, Joshi GP. Intraoperative airway management considerations for adult patients presenting with tracheostomy: A narrative review. Anesth Analg. 2021;132(4):1003-1011.
7. Dawson D. Essential principles: Tracheostomy care in the adult patient. Nurs Crit Care. 2014;19(2):63-72.
8. McGrath BA, Bates L, Atkinson D, Moore JA; National Tracheostomy Safety Project. Multidisciplinary guidelines for the management of tracheostomy and laryngectomy airway emergencies. Anaesthesia. 2012;67(9):1025-1041.
9. Hameed AA, Mohamed H, Al-Mansoori M. Acquired tracheoesophageal fistula due to high intracuff pressure. Ann Thorac Med. 2008;3(1):23-25.
10. Cipriano A, Mao ML, Hon HH, et al. An overview of complications associated with open and percutaneous tracheostomy procedures. Int J Crit Illn Inj Sci. 2015;5(3):179-188.
11. Heninger J, Ghosh A, Rowland M, et al. Accidental tracheostomy decannulation: Risk factors and complications in pediatric patients using the NSQIP-P database. Int J Pediatr Otorhinolaryngol. 2024;187:112174.
12. Cherches A, Wang A, Patterson RH, et al. Preventing pediatric accidental decannulation events: A quality improvement initiative. Int J Pediatr Otorhinolaryngol. 2024;183:112052.
13. Omokanye HK, Dunmade AD, Segun-Busari S, et al. Accidental decannulation of tracheostomy tubes — case series. J West Afr Coll Surg. 2016;6(1):108.
14. Meyers K, Burke Q, Siddiqui A, et al. Tracheostomy tube monitoring accessory to detect accidental decannulation and obstruction emergencies in ventilator-independent pediatric patients. Tracheostomy. 2024;1(3):7-16.
15. Zhou T, Wang J, Zhang C, et al. Tracheostomy decannulation protocol in patients with prolonged tracheostomy referred to a rehabilitation hospital: A prospective cohort study. J Intensive Care. 2022;10(1):34.
16. Singh RK, Saran S, Baronia AK. The practice of tracheostomy decannulation—a systematic review. J Intensive Care. 2017;5:1-12.
17. Lee M, Wilson H. Complications of tracheostomy. Shanghai Chest. 2021;5:42.
18. Yoo B, Lee B, Park JD, et al. Prevention of tracheo-innominate artery fistula formation as a complication of tracheostomy: Two case reports. Children (Basel). 2022;9(11):1603.
19. O’Malley TJ, Jordan AM, Prochno KW, et al. Evaluation of endovascular intervention for tracheo-innominate artery fistula: A systematic review. Vasc Endovascular Surg. 2021;55(4):317-324.
20. Alsunaid S, Holden VK, Kohli A, et al. Wound care management: Tracheostomy and gastrostomy. J Thorac Dis. 2021;13(8):5297.
21. Toeg H, French D, Gilbert S, Rubens F. Incidence of sternal wound infection after tracheostomy in patients undergoing cardiac surgery: A systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2017;153(6):1394-400.e7.
22. Kumarasinghe D, Wong E, Duvnjak M, et al. Risk factors associated with microbial colonisation and infection of tracheostomy tubes. Am J Otolaryngol. 2020;41(4):102495.
23. Skoretz SA, Anger N, Wellman L, et al. A systematic review of tracheostomy modifications and swallowing in adults. Dysphagia. 2020;35:935-947.
24. Goff D, Patterson J. Eating and drinking with an inflated tracheostomy cuff: A systematic review of the aspiration risk. Int J Lang Commun Disord. 2019;54(1):30-40.
25. Wallace S, McGrath B. Laryngeal complications after tracheal intubation and tracheostomy. BJA Educ. 2021;21(7):250-257.
26. Marvin S, Thibeault SL. Predictors of aspiration and silent aspiration in patients with new tracheostomy. Am J Speech Lang Pathol. 2021;30(6):2554-2560.
27. Hung J-J, Hsu H-S, Huang C-S, Yang K-Y. Tracheoesophageal fistula and tracheo-subclavian artery fistula after tracheostomy. Eur J Cardiothorac Surg. 2007;32(4):676-678.
28. Kim SP, Lee J, Lee SK, Kim DH. Surgical treatment outcomes of acquired benign tracheoesophageal fistula: A literature review. J Chest Surg. 2021;54(3):206.
29. James P, Parmar S, Hussain K, Praveen P. Tracheal stenosis after tracheostomy. Br J Oral Maxillofac Surg. 2021;59(1):82-85.
30. Shin B, Kim K, Jeong B-H, et al. Clinical implications of differentiating between types of post-tracheostomy tracheal stenosis. J Thorac Dis. 2017;9(11):4413.
31. Sriboonyong T, Preutthipan A, Nugboon M. Long-term sleep apnea CPAP via tracheostomy in children with tracheomalacia: 20-year experience. Front Pediatr. 2023;11:1169613.
32. McClintock C, McAuley DF, McIlmurray L, et al. Communication in critical care tracheostomy patients dependent upon cuff inflation: A scoping review. Aust Crit Care. 2024;37:971-984.
33. Veder LL, Joosten KFM, Zondag MD, Pullens B. Indications and clinical outcome in pediatric tracheostomy: Lessons learned. Int J Pediatr Otorhinolaryngol. 2021;151:110927.
34. Muller RG, Mamidala MP, Smith SH, et al. Incidence, epidemiology, and outcomes of pediatric tracheostomy in the United States from 2000 to 2012. Otolaryngol Head Neck Surg. 2019;160(2):332-338.
35. Dal’Astra APL, Quirino AV, Caixêta JA, Avelino MAG. Tracheostomy in childhood: Review of the literature on complications and mortality over the last three decades. Braz J Otorhinolaryngol. 2017;83(2):207-214.
36. Newton M, Johnson RF, Wynings E, et al. Pediatric tracheostomy-related complications: A cross-sectional analysis. Otolaryngol Head Neck Surg. 2022;167(2):359-365.
37. Morrison JM, Hassan A, Kysh L, et al. Diagnosis, management, and outcomes of pediatric tracheostomy-associated infections: A scoping review. Pediatr Pulmonol. 2022;57(5):1145-1156.
38. Russell CJ, Mack WJ, Schrager SM, Wu S. Care variations and outcomes for children hospitalized with bacterial tracheostomy-associated respiratory infections. Hosp Pediatr. 2017;7(1):16-23.
39. Doherty C, Neal R, English C, et al. Multidisciplinary guidelines for the management of paediatric tracheostomy emergencies. Anaesthesia. 2018;73(11):1400-1417.