
The RSV Turning Point: Implementing Preventive Tools in Everyday Practice
July 1, 2025
By Trahern W. Jones, MD
Executive Summary
This issue provides a comprehensive overview of respiratory syncytial virus (RSV), emphasizing its significant effect on infants and older adults and outlining recent advancements in prevention. It details the clinical burden of RSV, diagnostic and treatment limitations, and new immunization strategies, including vaccines for older adults and pregnant women and monoclonal antibodies such as nirsevimab for infants.
- As of 2023-2024, three U.S. Food and Drug Administration (FDA)-approved vaccines (Arexvy, Abrysvo, and mResvia) are available for adults ≥ 60 years of age; Abrysvo also is approved for pregnant individuals at 32-36 weeks of gestation to provide passive immunity to newborns.
- A single dose of nirsevimab is recommended for all infants in their first RSV season if the mother did not receive the RSV vaccine, with repeat dosing for select high-risk infants (e.g., chronic lung disease, immunocompromised, Alaska Native/American Indian) in their second season.
- Open, empathetic discussions, especially with vaccine-hesitant patients, are crucial. Misconceptions (e.g., concerns about heavy metals or fetal tissue use in vaccines) often can be corrected by reviewing product inserts and sharing scientific facts.
- RSV causes 58,000 to 80,000 hospitalizations annually in U.S. children younger than 5 years of age and even more in adults older than 60 years of age. The defined RSV season in North America runs from October through March.
- There is no antiviral cure; management includes supportive care. Corticosteroids, bronchodilators, and ribavirin generally do not improve outcomes in pediatric RSV bronchiolitis.
- Reverse transcription-polymerase chain reaction is the preferred diagnostic method for RSV, often available in multiplex respiratory panels. Rapid antigen tests are less sensitive.
- Adults aged 60 years and older or those 50 years of age or older with high-risk conditions, and pregnant people meeting specific timing criteria, are eligible for vaccination. The duration of immunity is under study but may last up to two years or more.
Case 1
It is October, and a previously healthy 30-year-old G1P0 female patient presents to your office at 32 weeks of gestation. Thus far, the pregnancy has been uncomplicated, and all prenatal testing has been normal or unremarkable. However, she has previously refused a tetanus-diphtheria-pertussis (Tdap) booster in this pregnancy, and she is skeptical about receiving the respiratory syncytial virus (RSV) vaccine. Specifically, she is worried that the vaccine contains heavy metals, such as mercury, that could injure her or her unborn child. In addition, she states that she has heard the vaccine was made from cell lines procured from aborted fetal tissue; thus, to receive the product would be incongruent with her religious beliefs. She says she has obtained all of this information from trusted sources in social media. However, her family has known you for many years, and she is interested in hearing your opinion today.
Case 2
A 14-month-old boy presents with his mother to your office in December. The child has a history of prematurity, born at 27 weeks, with complications, including patent ductus arteriosus (status post-closure) and chronic lung disease. The child was weaned off supplemental oxygen five months ago, and he is doing well. He is up-to-date on all of his immunizations so far. You note that his mother did not receive a dose of the RSV vaccine during the pregnancy. However, he did receive a dose of nirsevimab prior to his discharge from the neonatal intensive care unit 11 months ago. On review of his growth charts, you determine his weight is in the 8th percentile and his length is in the 12th percentile. He follows with speech and occupational therapy for oral aversion and mildly delayed milestones, but otherwise he is well. His mother asks for your guidance today regarding RSV prevention.
Introduction
RSV is a major cause of hospitalization, morbidity, and mortality in young children and older adults globally. Pediatricians are familiar with the yearly nuisance of “RSV season,” recognizing that periods of high transmission lead to packed wards, busy intensive care units (ICUs), and frequent discharges on home oxygen. Likewise, for the adult physician, there is increased recognition that periods of heightened RSV transmission lead to serious hospitalization attack rates among older and infirm adults. For many years, clinicians bemoaned the lack of substantive preventive steps beyond simple handwashing and respiratory droplet precautions.
In 1998, a monoclonal antibody directed against the surface fusion protein of the virus was introduced as a form of prophylaxis for young infants. Known as palivizumab (Synagis), it required monthly injections throughout the RSV season, and, because of its prohibitive cost, it was reserved for the highest-risk children. More recently, in 2023, a newer monoclonal antibody known as nirsevimab (Beyfortus) was introduced. A single injection could provide protection throughout the entire season and, overall, it represents a much more cost-effective medication.
In addition, 2023 also heralded the introduction of RSV vaccines for pregnant women and older adults. Similar in rationale to the familiar Tdap booster, the RSV vaccine for pregnant women induces the production of antibodies that cross the placenta and protect the infant for months after delivery. Likewise, the RSV vaccine for older adults provides a level of protection that was not possible previously.
This review will provide the primary care provider an overview of RSV disease in adults and children, with a special focus on these new preventive measures and considerations for their administration.
Epidemiology
Globally, RSV disease leads to approximately 3.6 million hospitalizations and 100,000 deaths each year in children younger than 5 years of age.1 Although quite common in older adult populations, the global burden of disease in this age group remains unclear. However, among high-income countries, some studies estimate that up to 470,000 hospitalizations and 33,000 in-hospital deaths of people older than 60 years of age can be attributed to RSV disease.2
Within the United States alone, RSV is believed to drive 58,000 to 80,000 hospitalizations per year among children younger than 5 years of age.3 Although often viewed as a pediatric ailment, notably this figure is much higher among adults — indeed, it is estimated that RSV infections are responsible for 100,000 to 150,000 hospitalizations in those older than 60 years of age each year in the United States.
While most cases are only mildly symptomatic, populations uniquely at risk for serious RSV disease include premature infants, children born with congenital heart disease, chronic lung disease (particularly bronchopulmonary dysplasia in premature infants or chronic obstructive pulmonary disease in older adults), genetic or chromosomal syndromes, and immunocompromising conditions, especially solid and hematopoietic transplant patients, as well as individuals older than 75 years of age.4,5
The moniker “RSV season,” familiar to many pediatricians, typically describes an annual surge in hospitalized cases beginning in late fall and ending in mid-spring in temperate climates.4 For the purposes of RSV vaccination and immunoprophylaxis, RSV season generally is defined as October through March. This yearly pattern was disrupted by the COVID-19 pandemic and associated school shutdowns and social distancing, leading to far fewer cases than normal in the 2020-2021 and 2021-2022 seasons. However, after a major surge in cases beyond historical norms in the 2022-2023 season, the pattern has started to realign again with historical trends in 2023-2024 and 2024-2025.4,6 (See Figure 1.)
Figure 1. Weekly Rates of RSV-Associated Hospitalizations by Season6 |
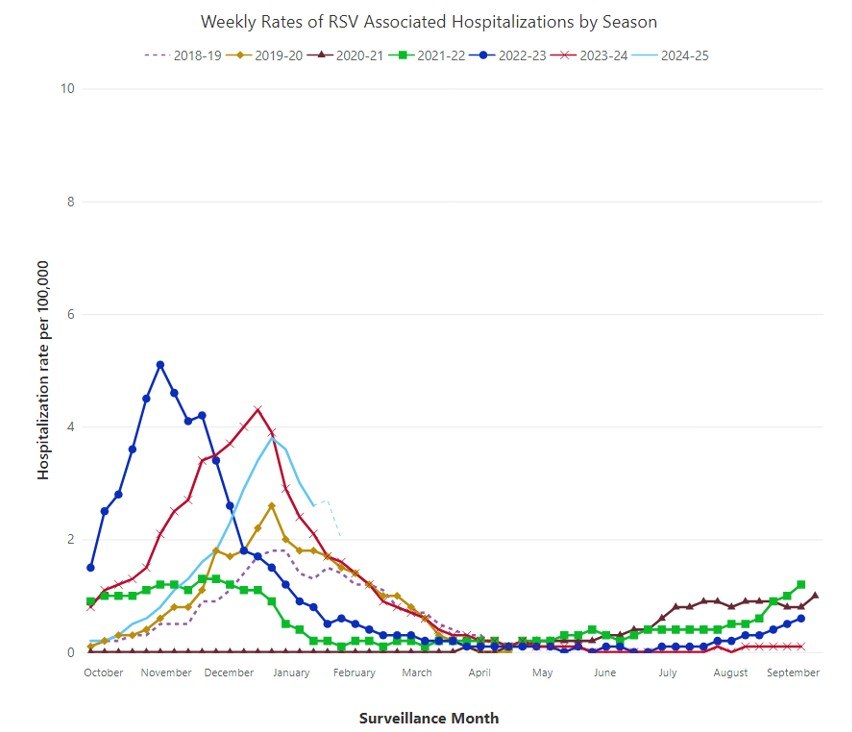 |
RSV: respiratory syncytial virus Source: Centers for Disease Control and Prevention. RSV-NET. Oct. 10, 2024. https://www.cdc.gov/rsv/php/surveillance/rsv-net.html |
RSV Pathophysiology
RSV is an enveloped ribonucleic acid (RNA) virus for which human beings are the only known natural reservoir.4 Viral isolates are subdivided into two major antigenic groups, labeled A and B, with about 80% nucleotide identity between them and substantial variation in some expressed glycoproteins.7 The most important glycoprotein for clinical purposes is known as the fusion protein F, which is responsible for facilitating viral entry into host cells. This is the target protein for all available vaccines, as well as the monoclonal antibodies palivizumab and nirsevimab, which function by binding protein F and disrupting viral entry into host cells.8,9
Normally, RSV is transmitted by inhalation or mucosal membrane contact with large-droplet aerosols produced by sneezing and coughing.10 Historical volunteer studies also have demonstrated that infection may occur by touching surfaces contaminated by respiratory secretions followed by contact with host mucosal membranes.11 After being in contact with host epithelial cells of the mucous membranes, the virus undertakes fusion (via protein F) with the cell membrane and begins entry and viral replication. The virus then may spread via cell-to-cell pathways or by microdroplet aspiration into the lower respiratory tract.
The incubation period from inoculation to symptomatic disease comprises anywhere from two to eight days, with four to six days being most common.4 As infection progresses, the host airways experience an inflammatory cascade with recruitment of mononuclear cells to the peribronchiolar space, along with necrosis of the small airway epithelia, followed eventually by congestion and plugging of the small airways by necrotic cells, fibrin, and leukocytes, compounded by epithelial edema.10 This cascade causes mechanical plugging of the host bronchioles, leading to respiratory distress and hypoxia. Coinfection with other respiratory viruses is frequent, and may compound such airway congestion and edema.
Symptomatology, Bronchiolitis, and RSV Disease
Many adult and pediatric patients will only experience mild symptoms of RSV infection. These generally include the “common cold” manifestations seen in most upper respiratory viral infections, including low-grade fevers, nasal congestion, scratchy throat, cough, and mild malaise or fatigue.7
However, as described earlier, more severe cascades of inflammation, congestion, epithelial edema, and airway plugging may lead to the classic syndrome of bronchiolitis seen in infants and toddlers. As the disease moves into the bronchioles and lower respiratory tract, infants may experience worsening cough, increased respiratory rate, dyspnea, and chest wall retractions that herald the onset of airway plugging.10
Such retractions in young infants are recognized by observing the costal margins, the supraclavicular region, and the suprasternal notch. The imbalance of the internal negative pressure from the infant’s respiratory effort vs. the external atmospheric pressure, combined with the inability of air to flow quickly through the small airways to equalize these forces, leads to a “sucking in” of the skin and soft tissues at these sites, in time with respiratory efforts. Such manifestations are named “retractions.” In addition, nasal flaring and head bobbing (sometimes also called “tracheal tugging”) also are signs of severe respiratory distress in young infants.
On auscultation, providers commonly hear diffuse and polyphonic wheezes throughout the patient’s lung fields. These sounds denote the high-pitched pressure differentials of air passing through edematous or congested airways. In addition, diffuse crackles and rhonchi signal the buildup of sloughed mucus and airway epithelia, as well as alveolar atelectasis and collapse as the result of airway obstruction.
Radiographic findings in bronchiolitis often are nonspecific. These include peribronchial cuffing caused by airway edema, along with sporadic hyperinflation or diaphragmatic flattening.9 Indeed, unless secondary complications (see the following sections) are suspected, chest radiography frequently is discouraged in infant bronchiolitis.12
As the disease and airway dysfunction of bronchiolitis worsen, children will experience worsening hypoxia, apnea, and respiratory failure. Additional complications in pediatric RSV bronchiolitis include dehydration caused by substantial insensible losses and inability to feed, as well as secondary bacterial pneumonia. Once thought to be rare, secondary bacterial pneumonia has been shown to be more common than previously thought, especially in premature infants or those with chronic lung disease.10 Isolated pathogens in these complications may include Streptococcus pneumoniae or nontypeable Haemophilus influenzae. Other complications in infant bronchiolitis can include urinary tract infection (although there is some consideration that this may be coincidental or nonpathogenic bacteruria) and pneumothorax as the result of high-flow oxygen treatment.10
Adults with severe RSV disease similarly may experience worsening cough, wheezing, and the appearance of lower lobe infiltrates or bronchial thickening on radiography.7,13 Secondary bacterial pneumonia rates in older adults may be quite high; studies demonstrate up to 10% to 20% of patients may experience a secondary bacterial pneumonia in hospitalized older adults, usually as the result of Streptococcus pneumoniae, Haemophilus influenzae, or Staphylococcus aureus.7,13 Rarely, other complications, such as cardiac arrhythmia, have been reported in older adults with RSV disease.
Reinfection after RSV disease is quite common, suggesting immunity from natural infection is not durable.14 In one study, up to half of adult volunteers experienced reinfection when challenged with an intranasal inoculation of RSV only two months after initial infection, and most were symptomatic with viral shedding.
Diagnostic Testing
RSV infection typically is diagnosed through molecular diagnostic testing with reverse transcriptase-polymerase chain reaction (RT-PCR) assays.4 These often are commercially available in combined multiplex assays for other common respiratory viruses, allowing providers to test for multiple agents from a single nasopharyngeal specimen. Rapid diagnostic tests, which detect viral antigen through direct fluorescent antibody or other forms of immunoassay, often are less sensitive than molecular diagnostic testing. Testing for acute and convalescent antibody seroconversion is not performed for clinical purposes but rather reserved for epidemiology studies. Seroconversion does not reliably occur in infants following RSV infection.4
Treatment
Supportive care, supplemental oxygen, respiratory support, and appropriate hydration and nutrition remain the keystones of therapy in RSV disease.12 Patients always should be placed in respiratory droplet isolation.
In the past, numerous other treatments have been attempted with little success in improving outcomes among children with RSV bronchiolitis. Although most of these have had little positive effect, some may be harmful. For example, the well-meaning use of corticosteroids as a means of decreasing inflammation has not been shown to improve short-term outcomes in infants with bronchiolitis; rather, they have been demonstrated to prolong viral shedding, which could pose a public health hazard.12 Bronchodilators (such as albuterol) have not been definitively shown to improve hospital stays. Similarly, other pharmacologic interventions intended to “open” the airways, such as the use of epinephrine or phenylephrine, have produced no positive effect and are not routinely recommended.
Other adjunctive treatments, such as chest physiotherapy, likewise have not shown clear benefit. It should be noted that superficial nasal suctioning is a reasonable and relatively harmless practice to reduce symptoms of nasal congestion; however, deep suctioning into the nasopharynx is hypothesized to only irritate inflamed mucosal tissues, and it has been shown to increase the length of hospital stay.12,15
Aerosolized ribavirin has been used as an antiviral therapy for RSV bronchiolitis.10 Although some studies have shown modest improvement in oxygen saturation with this medication, it has not clearly demonstrated improvement in mortality, need for mechanical ventilation, ICU stay, or other significant outcomes. Because of its lack of clearly proven efficacy, as well as its difficulty in administration, high cost, and teratogenic hazard to healthcare workers, aerosolized ribavirin is rarely used in the treatment of RSV bronchiolitis. If it is used, it usually is only among the seriously immunocompromised.
Because RSV is a viral infection, antibiotics have no efficacy against RSV bronchiolitis.12 However, if there is strong suspicion for secondary bacterial pneumonia in pediatric or adult patients, it is reasonable to start an empiric regimen that targets common pathogens such as methicillin-susceptible or methicillin-resistant Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, or Haemophilus influenzae (type A or nontypeable, and occasionally type B in unimmunized children).
Monoclonal antibodies (discussed later in this review) are not recommended or routinely used for treatment of active RSV disease in any age group.
RSV Vaccination
As a result of the relative paucity of pharmacologic therapies available to adult and pediatric patients, as well as the significant morbidity, mortality, and societal costs of yearly RSV infections, primary prevention of RSV infection and protection against severe disease has long been a goal of the public health and research community.
The history of RSV vaccine development extends back several generations of physicians and researchers for more than 50 years. The first RSV vaccine trials were undertaken in 1966, using an alum-precipitated, formalin-inactivated vaccine among seronegative children 2 months of age or older.10 In spite of a good antibody response to the vaccine, the project ultimately failed. Upon natural exposure to RSV, these children not only did not experience protection against infection, but rather experienced unexpectedly severe disease.16 Nearly 80% of the children in that cohort were hospitalized, and two of the 219 vaccine recipients died. It subsequently was hypothesized that the vaccines had elicited an overly exuberant T-cell response, leading to the markedly severe reaction when the children were naturally infected with RSV.
Ensuing vaccine development explored a variety of pathways and sought a more balanced immune response. Most recently, vaccines have targeted the viral F protein (responsible for fusion and entry of RSV into epithelial cells) with good success, and without the adverse immune enhancement seen in prior vaccine candidates. By exposing vaccine recipients to adjuvanted F protein in its pre-fusion conformation, an appropriate antibody response was elicited to prevent RSV infection and protect against severe RSV disease.17-20 Moreover, the antibodies induced from vaccination in pregnant women could be passed transplacentally to the fetus, providing post-natal protection for the newborn infant. It should be noted that the vaccine induces neutralizing antibodies against pre-fusion F protein, while natural infection induces antibodies against non-pre-fusion F protein.
As of this writing, the U.S. Food and Drug Administration (FDA) now has approved two protein-F vaccines for older adults in the United States: Pfizer’s Arexvy and GSK’s Abrysvo. Each is a recombinant stabilized pre-fusion F protein vaccine.4 Arexvy’s recombinant F protein is based on the RSV-A subtype, while Abrysvo’s is based on both RSV-A and -B subtypes.21 Arexvy is approved for all adults age 60 years and older, as well as adults age 50 years and older at higher risk for lower respiratory tract disease.22 Abrysvo is approved for all adults age 60 years and older, as well as adults age 18 through 59 years at higher risk for lower respiratory tract disease and pregnant people.23-25 Both vaccines have been shown to have an efficacy of 67% to 83% against RSV lower respiratory tract disease in adults older than 60 years of age.17,18 Adults with high-risk conditions for lower respiratory tract disease include those with chronic heart or lung disease, immunocompromising conditions, end-stage renal disease, diabetes mellitus complicated by end-organ damage or requiring insulin or sodium-glucose cotransporter-2 (SGLT2) inhibitor treatment, neurologic or neuromuscular disease leading to impaired airway clearance, chronic hematologic disease, or those who live in a nursing home.26 (See Table 1.)
Table 1. Indications and Eligibility for Adult RSV Vaccination23,24 |
Adults < 60 Years of Age |
Abrysvo may be given to adults ages 18-59 years and Arexvy may be given to adults ages 50-59 years if any of the following high-risk conditions are present:
|
Adults ≥ 60 Years of Age |
Abrysvo, Arexvy, and mResvia may be given to any adult 60 years of age or older |
Pregnant People |
Only Abrysvo may be given under the following conditions:
|
RSV: respiratory syncytial virus |
Side effects of the Arexvy and Abrysvo vaccines are relegated largely to reactions at the site of administration, including pain, redness, and swelling.23 Additional side effects can include headache, fatigue, fever, nausea, diarrhea, and muscle or joint pain. Rarely, Guillain-Barré syndrome (GBS) has been reported among vaccine recipients; most recent estimates place this at fewer than 10 cases per 1 million older adults who receive either the Arexvy or Abrysvo vaccines.23 Both vaccines had several reports of atrial fibrillation within 30 days among vaccine recipients and placebo recipients; the data available from these trials were insufficient to determine if a causal relationship could be established.19,20
In 2024, Moderna’s mResvia became commercially available as an additional FDA-approved vaccine for older adults. This vaccine uses lipid nanoparticles to deliver a messenger RNA (mRNA)-encoded pre-fusion F protein into the recipient’s intramuscular tissue.27 Similar to the better-known SARS-CoV-2 mRNA vaccines in concept, mResvia’s mRNA delivery system uses the recipient’s ribosomes to produce RSV pre-fusion F protein, which the immune system subsequently recognizes as pathogenic and develops an antibody response against. In clinical trials, the mRNA-based mResvia vaccine has demonstrated an efficacy rate of about 83% against RSV lower respiratory tract disease in adults older than 60 years of age.28 However, efficacy over a longer study period of 18 months has been observed to drop to 50%, which is significantly lower than that observed in the recombinant vaccines.29 Current indications are the same as for the adjuvanted protein-F vaccines mentioned earlier. (See Table 1.) Likewise, side effects include reactions at the site of administration, as well as headache, fatigue, fever, nausea, diarrhea, and muscle or joint pain.23 GBS has not been observed with mResvia at the time of this writing.
Pfizer’s Abrysvo can be given as a single intramuscular dose to pregnant people between 32 and 36 weeks of gestation.24 (See Table 1.) The vaccine is recommended to be given in this time frame between September and January in North America, to coincide with the newborn infant’s anticipated exposure to community RSV circulation. When given in this fashion, vaccine efficacy in clinical trials was estimated at 82% for protection against RSV lower respiratory tract disease in newborns within 90 days after birth, and 70% within 180 days after birth.4 Safety profiles between placebo and vaccine were similar, although a slightly higher (but statistically nonsignificant) proportion of premature births was seen in vaccine recipients compared to placebo recipients; notably, premature birth sometimes was seen in vaccine recipients more than 30 days after vaccine receipt, suggesting there may not have been a true pathophysiologic association.4
If a pregnant person already has received an RSV vaccine during at least one prior pregnancy, they are not recommended to undergo repeat immunization in future pregnancies.24 However, as the vaccine’s efficacy is better studied over time and the question of how much immunity wanes for these populations is answered, this recommendation may be adjusted.
It is unknown how long immunity from RSV vaccination may last in adults, but initial data suggest that one dose may prevent infection or provide protection against severe disease for up to two years or longer.23
Monoclonal Antibodies
Although pediatric vaccines currently are not available, protection against RSV disease can be gained through the injection of monoclonal antibodies similarly directed against the F protein necessary for viral entry into epithelial cells. As the antibodies are naturally metabolized, serologic protection wanes and repeat doses are necessary. These products generally are targeted for newborns or older infants with specific high-risk conditions (see the following paragraphs).
The first monoclonal antibody introduced for this purpose was palivizumab (Synagis). This immunoprophylaxis regimen required monthly injections during RSV season to maintain serologic protection against RSV.9 Over its tenure, palivizumab was found to reduce hospitalizations caused by RSV but had little effect on mortality or adverse events.30 Significantly, the cost of this medication was especially burdensome for families and healthcare systems. Among premature infants, immunoprophylaxis with palivizumab could cost between $1,600 and $2,600 per month during RSV season, and the cost per prevention of one hospitalization was estimated at more than $300,000.31 At this time, palivizumab generally is recommended only if newer monoclonal antibody products (see the following sections) are not available.
More recently, nirsevimab (Beyfortus) has been introduced as a new monoclonal antibody with significant advantages over palivizumab. Importantly, nirsevimab’s half-life in the human body is much longer than its predecessor; thus, only one injection is required for coverage during the entire RSV season.4 Because of this, as well as its lower cost per dose (ranging from $500-$1,040, depending on the dose size), nirsevimab represents a much more cost-effective medication for RSV immunoprophylaxis.32 In terms of its clinical advantages, nirsevimab has been demonstrated to have a pooled efficacy of 79% in preventing serious lower respiratory tract disease caused by RSV, an efficacy of 81% in preventing hospitalization caused by RSV, and an efficacy of 90% in preventing ICU admission caused by RSV.4
Unlike palivizumab, nirsevimab is universally recommended for all infants in the first RSV season, with some nuance depending on their birthing parent’s receipt of the RSV vaccine.33 (See Table 2.) Essentially, all infants are recommended to receive nirsevimab if the following conditions are met:
- The infant is 8 months of age or younger, born in or entering their first RSV season.
- The mother did not receive the RSV vaccine during pregnancy.
- The mother’s RSV vaccination status is unknown.
- The mother received the RSV vaccine 14 days or less before the delivery.
Table 2. Indications and Eligibility for Pediatric Nirsevimab Immunoprophylaxis33 |
First RSV Season |
|
Second RSV Season |
|
RSV: respiratory syncytial virus |
For these conditions, “RSV season” is defined as October through March in North America. If the infant is hospitalized for a prolonged period after delivery (i.e., neonatal intensive care patients), the infant should receive their dose of nirsevimab shortly before or just after discharge.4 Administration of nirsevimab while hospitalized is not recommended at this time.
Infants entering their second RSV season (between the ages of 8 and 19 months) may qualify for a dose of nirsevimab if they have a high-risk condition for severe RSV disease.33 (See Table 2.) These include:
- Children with chronic lung disease as the result of prematurity AND who required supplemental oxygen, diuretic therapy, or chronic corticosteroid therapy within the past six months leading up to the start of the SECOND RSV season;
- Children with severely immunocompromising conditions;
- Children with cystic fibrosis AND who have severe lung disease, including hospitalization for pulmonary exacerbation(s) in the first year of life, or with abnormalities on chest imaging when stable AND/OR weight-for-length < 10th percentile;
- American Indian or Alaska Native children.
For all of the these situations, children should receive a dose of nirsevimab according to these recommendations for the first or second season regardless of any history of natural RSV infection or reinfection.4 Also of note, children who have undergone cardiopulmonary bypass should receive an additional dose of nirsevimab after surgery during RSV season, if eligible based on age and regardless of whether the mother received the RSV vaccine. This is because of the likely loss of the child’s own or transplacentally acquired antibodies when undergoing this procedure. Likewise, extracorporeal membrane oxygenation (ECMO) also would lead to loss of maternal antibodies, and providers should consider repeating a dose of nirsevimab afterward.34
Nirsevimab is dosed according to a weight-band schema.4 Infants < 5 kg should be given 50 mg, and infants ≥ 5 kg should receive 100 mg. Children 8 to 19 months of age should receive 200 mg administered as two separate 100-mg doses.33
Talking to Patients About Vaccines and Immunoprophylaxis
Numerous misconceptions about vaccines and immunoprophylaxis abound in modern media. Although such problems are legion, to cover each one would exceed the scope of this review. Indeed, many such misconceptions feel far beyond the ability of many providers to change or fix. However, providers should bear in mind some key points when recommending vaccines to their patients and families.
First, trust and personal connections often are extremely important in individual decision-making about vaccines.35 Patients should feel comfortable asking their provider questions in an office setting, and open discussion should be encouraged. From the author’s own personal perspective, providers should speak openly about their own families and decision-making to improve their approachability in this regard. If the provider has a child, it often is helpful to explain if their own child has received the vaccine or immunoprophylaxis in question.
Second, providers should understand that their own consistent recommendations and reminders for vaccination are one of the most important factors in this decision-making process for patients. One study demonstrated that the greatest proportion of vaccine-skeptical patients who changed their minds about vaccination listed “information or assurances from healthcare provider” as the chief reason.35
Third, providers should personally strive to better understand the vaccine products they recommend, and they should understand the importance of vaccination to maintain personal health. Studies have demonstrated that health professionals’ own knowledge and attitudes toward vaccines are associated with improved recommendation practices in their patient population.36 Moreover, providers should feel comfortable discussing the benefits vs. risks of vaccination, indications and contraindications, and even some of the common misconceptions surrounding vaccines and immunoprophylaxis.
Finally, humanistic relationships with vaccine-hesitant or vaccine-skeptical patients should continue to be cultivated. For patients who experience only mild vaccine hesitancy, one or two visits with a thoughtful, conversational approach often is reassuring and improves vaccine uptake. However, many patients who feel strongly skeptical about vaccines will refuse to vaccinate despite the physician’s best efforts — yet, perplexingly, they will continue to seek care with the physician. The physician should not take such behaviors personally but should recognize that the patient still is interested in hearing the healthcare provider’s opinions, even if they disagree with them. This can be a valuable route for communication in future visits, and breaking the connection with this patient may lead to them seeking more extreme opinions elsewhere.
Conclusion
RSV remains a serious burden to global and North American health. Each year, many thousands of young infants experience prolonged hospitalizations and major complications because of the disease. Similarly, older and frail adults experience high rates of morbidity and mortality, as well as secondary bacterial pneumonia. Sadly, physicians are quite familiar with many of these problems. Many pediatric residents learn to grit their teeth and pass uncomplainingly through the crucible of “RSV season” each winter.
However, the year 2023 heralded remarkable new achievements in the prevention of RSV infection and protection against serious RSV disease. For the first time in history, the American public has gained access to efficacious and safe new RSV vaccines that can prevent serious morbidity, mortality, and hospitalizations for many millions of young and old alike. In addition, where neonates previously only had access to costly and relatively poorly efficacious monoclonal antibodies for protection of serious RSV disease, a newer product has become available that relieves major financial burdens and offers excellent protection.
Future work will be needed to determine how quickly immunity wanes over time, whether repeat vaccinations will be needed, and whether young infants would overall benefit more from immunoprophylactic strategies or vaccination strategies. In the meantime, primary care providers and nurses have gained another new tool to save their patients and families from the nuisance, pain, and grief of seasonal RSV disease — with great hope in the offing, such work has been passed on to their ready and capable hands.
Case 1: Conclusion
You sit down with your 30-year-old G1P0 patient to learn more about her beliefs and questions regarding vaccines. She explains that she has had strong feelings about politics in the past year and, consequently, she began to watch more videos on social media that expressed suspicion about the content of vaccines and the motivations of the companies that make them. When you ask about specific harms she is worried about, she voices that she is worried about the manufacturing process and possible heavy metal usage, as well as the use of aborted fetal tissue.
You welcome her questions and indicate that you also recently wanted to learn more about the newly available RSV vaccines. Together, you pull up the product insert on your office computer, which is freely available on the FDA website. Looking at the insert together, you find there are no aluminum adjuvants or other heavy metals listed, and the vaccine is preservative-free. In addition, the cell lines used to express the stabilized pre-fusion F protein are not derived from human fetal tissue but rather hamster ovary cells, and the vaccine protein is fully separated from these cell lines in the manufacturing process.
Finally, you discuss how important maternal transplacental antibodies can be for protecting her baby from RSV, and that you would recommend her receiving the vaccine today. She appreciates that you have listened carefully to her today, and indicates that you are well-known to her family and friends and that she trusts you. With that, she decides to receive the RSV vaccine after all.
Case 2: Conclusion
On review of the 14-month-old infant’s chart, you determine that he qualifies for another dose of nirsevimab for his second RSV season. Specifically, he qualifies because he has chronic lung disease because of prematurity, for which he was on supplemental oxygen within the past six months leading up to the second RSV season. You explain this information to his mother.
She asks if this is a vaccine or an antiviral medication. You explain that it is neither, but rather a monoclonal antibody, very similar to those produced naturally by the human immune system. She expresses gratitude for the information, and two doses of 100 mg nirsevimab (for a total of 200 mg) are prepared to be given to the infant today.
Trahern W. Jones, MD, is Assistant Professor, Pediatric Infectious Diseases, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City.
References
1. Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022;399(10340):2047-2064.
2. Savic M, Penders Y, Shi T, et al. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: A systematic literature review and meta-analysis. Influenza Other Respir Viruses. 2023;17(1):e13031.
3. Centers for Disease Control and Prevention. Surveillance of RSV. August 30, 2024. https://www.cdc.gov/rsv/php/surveillance/index.html
4. American Academy of Pediatrics. Respiratory syncytial virus. In: Kimberlin DW, Banerjee R, Barnett ED, et al, eds. Red Book: 2024-2027 Report of the Committee on Infectious Diseases. American Academy of Pediatrics;2024:713-721.
5. Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: Infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30(1):277-319.
6. Centers for Disease Control and Prevention. RSV-NET. October 10, 2024. https://www.cdc.gov/rsv/php/surveillance/rsv-net.html
7. Walsh EE, Englund JA. Respiratory syncytial virus. In: Bennett JE, Dolin R, Blaser MJ eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier;2020:2093-2103.
8. U.S. Food and Drug Administration. Beyfortus (nirsevimab-alip) prescribing information. Revised August 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761328s005lbl.pdf
9. U.S. Food and Drug Administration. Synagis (palivizumab) prescribing information. Revised March 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/103770s5185lbl.pdf
10. Welliver RC. Respiratory syncytial virus. In: Cherry JD, Kaplan SL, Harrison GJ, et al, eds. Feigin and Cherry s Textbook of Pediatric Infectious Diseases. 9th ed. Elsevier;2025:1865-1882.
11. Hall CB, Douglas RG Jr. Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99(1):100-103.
12. Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-1502. [Erratum in: Pediatrics. 2015;136(4):782].
13. Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13(3):371-384.
14. Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693-698.
15. Mussman GM, Parker MW, Statile A, et al. Suctioning and length of stay in infants hospitalized with bronchiolitis. JAMA Pediatr. 2013;167(5):414-421.
16. Chin J, Magoffin RL, Shearer LA, et al. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89(4):449-463.
17. Ison MG, Papi A, Athan E, et al; AReSVi-006 Study Group. Efficacy and safety of respiratory syncytial virus (RSV) prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons. Clin Infect Dis. 2024;78(6):1732-1744.
18. Walsh EE, Pérez Marc G, Zareba AM, et al; RENOIR Clinical Trial Group. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465-1477.
19. U.S. Food and Drug Administration. Arexvy (respiratory syncytial virus vaccine, adjuvanted) prescribing information. Revised May 2025. https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert-AREXVY.pdf
20. U.S. Food and Drug Administration. Abrysvo (respiratory syncytial virus vaccine) prescribing information. 2024. https://www.fda.gov/media/168889/download
21. Centers for Disease Control and Prevention. Healthcare providers: RSV vaccination for adults 60 years of age and over. July 3, 2024. https://www.cdc.gov/vaccines/vpd/rsv/hcp/older-adults.html
22. U.S. Food and Drug Administration. Arexvy. May 27, 2025. https://www.fda.gov/vaccines-blood-biologics/arexvy
23. Centers for Disease Control and Prevention. Vaccines for older adults. August 30, 2024. https://www.cdc.gov/rsv/vaccines/older-adults.html
24. Centers for Disease Control and Prevention. RSV vaccine guidance for pregnant women. August 30, 2024. https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/pregnant-people.html
25. U.S. Food and Drug Administration. Abrysvo. Feb. 21, 2025. https://www.fda.gov/vaccines-blood-biologics/abrysvo
26. Centers for Disease Control and Prevention. Clinical overview of RSV. August 30, 2024. https://www.cdc.gov/rsv/hcp/clinical-overview/index.html
27. Centers for Disease Control and Prevention. ACIP evidence to recommendations for use of Moderna RSV vaccine (mResvia) in all adults aged ≥ 75 years and in adults aged 60-74 at increased risk of severe RSV disease. Sept. 5, 2024. https://www.cdc.gov/acip/evidence-to-recommendations/mrna-rsv-vaccine-older-adults-etr.html
28. Wilson E, Goswami J, Baqui AH, et al. Efficacy and safety of an mRNA-based RSV preF vaccine in older adults. N Engl J Med. 2023;389(24):2233-2244.
29. Das R. Update on Moderna’s RSV vaccine, mResvia (mRNA-1345), in adults ≥ 60 years of age. ACIP presentation, June 26-28, 2024. Centers for Disease Control and Prevention. https://stacks.cdc.gov/view/cdc/157864
30. Garegnani L, Styrmisdóttir L, Roson Rodriguez P, et al. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst Rev. 2021;11(11):CD013757.
31. Hampp C, Kauf TL, Saidi AS, Winterstein AG. Cost-effectiveness of respiratory syncytial virus prophylaxis in various indications. Arch Pediatr Adolesc Med. 2011;165(6):498-505.
32. American Academy of Pediatrics. Nirsevimab (Beyfortus) product & ordering information. Updated July 11, 2024. https://www.aap.org/en/patient-care/respiratory-syncytial-virus-rsv-prevention/nirsevimab-beyfortus-product--ordering-information/
33. Centers for Disease Control and Prevention. RSV immunization guidance for infants and young children. Aug. 30, 2024. https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/infants-young-children.html
34. American Academy of Pediatrics. Nirsevimab (Beyfortus) frequently asked questions. Updated May 8, 2025. https://www.aap.org/en/patient-care/respiratory-syncytial-virus-rsv-prevention/nirsevimab-frequently-asked-questions/
35. Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: Which vaccines and reasons why. Pediatrics. 2008;122(4):718-725.
36. Fernandes A, Wang D, Domachowske JB, Suryadevara M. Vaccine knowledge, attitudes, and recommendation practices among health care providers in New York State. Hum Vaccin Immunother. 2023;19(1):2173914.