Preparing for Avian Influenza
May 1, 2025
Executive Summary
- Two highly pathogenic strains of avian influenza currently are described in significant circulation at the time of this writing — the H5 and H7 strains. The bulk of all animal and human infections of avian influenza currently is caused by an H5N1 strain. While both H5 and H7 strains are believed to have high pandemic potential, H7N9 is believed to be the most dangerous circulating highly pathogenic avian influenza (HPAI) in this regard.
- Human cases of HPAI H5N1 in the United States have been much more limited so far. Since January 2022, a total of 70 human cases have been confirmed in the United States, with one death occurring. The majority of these cases are suspected to have contracted their infection from working with infected commercial dairy cattle (41 cases), working with infected commercial poultry (24 cases), or working with other animals, such as backyard poultry flocks or wild birds (two cases). Three cases do not have a known source for their infection. No human-to-human transmission events have yet been recorded for HPAI H5N1 in the United States.
- Typically, influenza A viruses in humans require an incubation of one to four days from the time of exposure to the onset of symptoms. Most uncomplicated cases of human influenza A infection are characterized by sudden onset of systemic symptoms, such as fever, myalgias, chills, headaches, malaise, and anorexia. Respiratory symptoms usually include dry cough, pharyngitis, and nasal congestion and rhinorrhea.
- Of particular note, conjunctivitis and/or subconjunctival hemorrhage often have been features of the H5N1 strain in humans in the United States, frequently without any other respiratory or systemic symptoms.
- Currently, there are no commercially available tests to positively identify H5N1 virus, and most testing must be arranged through local health departments for specific situations. Currently, testing for HPAI H5N1 should not routinely be performed in most individuals. However, patients presenting with upper respiratory symptoms or conjunctivitis within 10 days of any high-risk exposures should undergo testing for HPAI H5N1 in coordination with local public health authorities.
- Neuraminidase inhibitors generally have been shown to be effective against HPAI strains, particularly H5N1. In particular, oseltamivir is recommended for promptly treating cases of confirmed or suspected HPAI H5N1 infection in humans.
Although human-to-human transmission has not been observed for avian influenza in the United States at this time, and the situation is seen only as a possibility, it is important that all emergency care providers understand the virus and its potential implications for both practice and patients.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
By Trahern Jones, MD
Case
A previously healthy 7-year-old girl presents to your facility with fever, cough, conjunctivitis, muscle aches, and shortness of breath. Routine polymerase chain reaction (PCR) viral testing of a nasopharyngeal swab reveals that she is positive for influenza A, but not the H1 or H3 strains. While discussing this with her mother, you discover that she works on a family farm, where she routinely is exposed to numerous free-range chickens and a small dairy cattle herd. In addition, she consumes unpasteurized milk from her family farm quite regularly. The mother also reports that a larger commercial farm in their county had an outbreak of H5N1 avian influenza with their flock of 10,000 chickens, and these recently had to be culled. She asks the provider what steps should be taken next to evaluate and treat her daughter.
Introduction
Highly pathogenic avian influenza (HPAI) viruses pose serious threats to global animal and human health. Two subtypes of HPAI currently pose serious pandemic potential (H5 and H7) and have caused multiple outbreaks in wild birds, domesticated poultry, and other animal species around the world for several decades.1
More recently, the H5N1 influenza A strain (associated with wild birds and poultry) has led to 70 human cases since 2022.2 In addition, hundreds of millions of poultry have been affected in the United States — as well as nearly 1,000 dairy herds. As a result, there now is serious concern that, eventually, there could be a broader human-to-human transmitted epidemic of HPAI H5N1 in North America.
However, it should be stressed that human-to-human transmission in the United States has not been observed at this time, and the situation is seen only as a possibility, albeit with serious public health implications. Moreover, while there are the previously noted concerns for human disease transmission, there already are serious stresses on North American agricultural supply chains.
This review will seek to explain the HPAI viruses in better detail to help pediatric healthcare providers understand and explain the ongoing situation to families and the public. The current epidemiologic and epizoologic situation for humans and wild and domesticated animals will be elucidated. Moreover, the necessary virology, pathophysiology, and clinical manifestations will be outlined. Lastly, practical steps in identification of potential cases, diagnosis, prevention, and treatment will be explained.
Virology
Influenza viruses are classified in three distinct types: influenza A, influenza B, and influenza C.3 While all three share basic characteristics (Orthomyxoviridae family, single-stranded ribonucleic acid [RNA] viruses that cause respiratory tract infections), influenza A is distinguished most importantly by its broad capacity to infect numerous species besides humans, which, consequently, raises its pandemic potential. On the other hand, influenza B is found only within humans, and influenza C is found only among humans and, occasionally, swine.
Furthermore, influenza A viruses are classified by the presence of unique antigens embedded within the viral envelope: hemagglutinin (HA) and neuraminidase (NA).3 The HA protein is responsible for viral attachment to host epithelial or other tissue cells. The NA protein catalyzes further steps in the viral entry process as well as release of viral particles from infected host cells. Inhibition of the NA protein is targeted by more commonly known antivirals specific for influenza A, like oseltamivir and peramivir. Overall, at least 16 HA proteins and nine NA proteins are known so far.3
Influenza A viruses often are referred to by their constituent types of HA and NA proteins. Examples include H1N1 (which was responsible for the 1918 pandemic flu and the 2009 swine flu), H3N2 (responsible for the 1968 Hong Kong pandemic), and H5N1 (one of the known HPAIs).3 Note that there still are genetic variations within the H and N types. For example, while many of the influenza A strains circulating today in the community are classified as H1N1, this does not mean they are genetically identical to the influenza strain that caused the 1918 pandemic. However, they are, in a simplified manner of speaking, descendents of that strain.
Small variations in the amino acid sequences in the antigenic sites on the HA and NA proteins can accumulate gradually over numerous viral generations (or over the course of numerous human or animal hosts).3 This leads to the phenomenon of antigenic drift, wherein host immunity loses efficacy against strains as variations in the antigenic site accumulate, thus changing the binding affinity of host antibodies in successive exposures. Because of this, an individual’s prior immunity (whether through immunization or natural disease) to a particular H-type N-type virus may wane in future seasons, although some protection still may be conferred via residual antibody binding.
More concerningly, antigenic shift occurs when rearrangement events in the genome of a given influenza A virus lead to the appearance of a new subtype. For example, this phenomenon is believed to have led to the introduction of the H1N1 1918 pandemic influenza, which led to catastrophic loss of human life on all inhabited continents.3 Human immunity to such strains often is limited or absent because of the novelty of the combined HA and NA proteins. Because of this, the virus is capable of rapidly spreading throughout communities with little or no ability to fight severe infection. Antigenic shift events are believed to occur especially commonly with interspecies transmission of influenza A viruses. Therefore, HPAI strains that infect humans or other mammals are considered very worrisome for their pandemic potential, and they remain a top priority in influenza research and pandemic planning.
Two highly pathogenic strains of avian influenza are currently described in significant circulation at the time of this writing — the H5 and H7 strains.2 The bulk of all animal and human infections of avian influenza currently is caused by an H5N1 strain. While both H5 and H7 strains are believed to have high pandemic potential, H7N9 is believed to be the most dangerous circulating HPAI in this regard.4
Epidemiology and Epizootiology
Cases of commonplace influenza A and influenza B in the northern hemisphere typically are transmitted and acquired from October through April.5 Peak numbers of infection often occur later in this timeframe — 58% of cases are contracted after February.5 Of course, the “opposite” pattern is true in the southern hemisphere. There, influenza season typically takes place from April through September, reflecting the winter months in those parts of the world. However, it is important to note that, in the tropics, influenza viruses circulate year-round.5
Taken together, more commonplace subtypes of Influenza A and Influenza B cause substantial morbidity and mortality in the United States each year. Most recently, in the 2023-2024 influenza season, 40 million cases are estimated to have occurred in the United States, leading to 470,000 hospitalizations and 28,000 deaths.6 The greatest morbidity and mortality usually concentrates among the very young (< 4 years of age) and the old (> 65 years of age).3 Some studies have estimated that three-quarters of seasonal influenza deaths occur among adults older than 65 years of age. Additional at-risk groups include the immunocompromised; pregnant people; adults and children with chronic respiratory disease (such as asthma or chronic obstructive pulmonary disease); and people with chronic cardiovascular disease, chronic kidney disease, and chronic liver disease. People who are obese, who have diabetes mellitus, and those living in long-term care facilities also are at higher risk. (See Table 1.)
Table 1. High-Risk Conditions that Predispose Patients to Severe Outcomes Caused by any Influenza Virus3 |
|
However, it also should be cautioned that even previously healthy people experience serious hospitalizations, intensive care unit admissions, and deaths each season, underlining the hazards of this otherwise commonplace disease. In fact, half of all recorded pediatric deaths attributable to influenza were in patients who did not have any preexisting comorbidities.4
The first outbreak of HPAI H5N1 was reported in a child in Hong Kong in 1997, which was associated with a cluster of 18 cases (11 of whom were children) before all live bird markets were closed and all poultry were culled on the island.7 HPAI H5N1 then was discovered in mainland China in 2003.
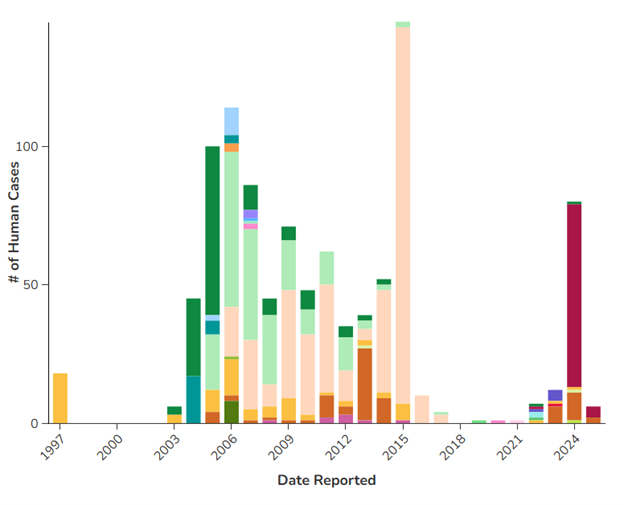
It has circulated or been found in various regions throughout the globe for the past two decades.8,9 (See Figure 1.) Most of these infections have occurred among wild birds, with occasional spread into poultry flocks and incidental human involvement. Limited human-to-human transmission has occurred in these situations abroad.
Figure 1. Past Reported Global Human Cases with Highly Pathogenic Avian Influenza A by Country |
 |
Centers for Disease Control and Prevention. Past reported global human cases with highly pathogenic avian influenza A (H5N1) (HPAI H5N1) by country, 1997-2025. Updated March 25, 2025. https://www.cdc.gov/bird-flu/php/avian-flu-summary/chart-epi-curve-ah5n1.html |
In January 2022, HPAI H5N1 was first described in the United States.10 Since then, this strain has had a substantial impact on domestic poultry and cattle production. More than 160 million poultry have been affected since 2022 in all regions of the United States.2 Deaths of these flocks, either because of the virus or culling activities performed to control its spread, have led to severe restrictions in the domestic egg supply.11 Among dairy cattle, nearly 1,000 herds have been affected in 17 different states.2
Human cases of HPAI H5N1 in the United States have been much more limited so far. Since January 2022, a total of 70 human cases have been confirmed in the United States, with one death occurring.2 The majority of these cases are suspected to have contracted their infection from working with infected commercial dairy cattle (41 cases), working with infected commercial poultry (24 cases), or working with other animals, such as backyard poultry flocks or wild birds (two cases).2 Three cases do not have a known source for their infection. No human-to-human transmission events have yet been recorded for HPAI H5N1 in the United States.2
Transmission
Influenza viruses usually are transmitted via respiratory routes or contact with mucous membranes.4 Droplets containing infective influenza virus are generated by coughing, sneezing, talking, or otherwise quiet breathing. Larger-diameter droplets usually will travel via ballistic trajectories from an infected host and land on other potential hosts or inanimate surfaces within a space of 1-3 meters.3,4 Smaller-diameter droplets, especially those generated by medical procedures, such as intubation, bronchoscopy, high-flow or airway pressure oxygen delivery devices, dental procedures, or suctioning, may remain suspended in air and can deliver a viable virus to potential hosts over longer distances.
In addition, indirect contact through surfaces and objects contaminated with influenza virus from respiratory droplets or secretions (fomites) also may be infectious for up to 24 hours.4 A viable virus is, thereafter, transferred by contact from these surfaces to host mucous membranes, leading to infection.
With specific regard to avian influenza, HPAI H5N1 is shed from the saliva, respiratory secretions, and feces of wild and domesticated birds.12 Viable virus loads also may contaminate surfaces, which can lead to fomite spread. Similar to the routes of entry of more everyday influenza strains, animal and human hosts become infected through direct contact and droplet transmission of secretions via the mucous membranes and respiratory tract. For agricultural workers and children in farm settings, direct handling and work among infected flocks may lead to infection via inhalation of respiratory droplets. In addition, handling contaminated feces, egg crates, cages, or other equipment and surfaces may pose a significant risk of transmission.11
Genetic material from HPAI H5N1 also has been detected in unpasteurized cow’s milk from infected herds.12 While no human cases of H5N1 have yet been traced to the consumption of unpasteurized cow’s milk, experimental evidence has demonstrated that both mice and cats are capable of infection through this route, suggesting that human consumption of unpasteurized milk could be a risk factor.
Pathophysiology
In general, influenza A viruses induce disease by largely similar pathways. Upon deposition of an infected respiratory droplet on mucous membranes or respiratory columnar epithelia, viral particles attach to host cells through the action of HA.3 This occurs through binding sialic acid residues of glycoproteins on the host cell surface. After cell entry, genetic material from the influenza virus enters the nucleus, facilitating viral replication using host cell machinery. Newly created virions then are issued to the cell surface, where NA cleaves binding sites with host cell glycoproteins, thus releasing a new generation of virus into the respiratory tract.8
Such appropriation of cellular components for production of virions leads to loss of essential cellular functions. Infected cells subsequently will undergo both necrosis and apoptosis, leading to destruction and inflammation of respiratory tract epithelia.3 Adjacent and nearby cells in the host epithelium subsequently will be infected. In particular, pneumonitis with diffuse alveolar damage may ensue, with viral penetration into the lower respiratory tract.8 Alveolar spaces may experience edema, fibrin deposition, hyaline membrane formation, and even hemorrhage.
HPAI strains of H5N1 and H7N9 have several unique considerations with regard to pathogenesis of disease in human beings. HA in these strains is hypothesized to have a special affinity for α-2,3-sialic acids commonly found in avian species and, coincidentally, in greater abundance in the human lower respiratory tract.3,13 This phenomenon could help explain the relative severity of HPAI disease when humans are infected.
However, it also could explain why humans are less frequently infected than avian species — the lack of α-2,3-sialic acids in the upper human respiratory tract makes HPAI viral binding more difficult. In addition, HPAI strains are believed to favor viral replication at higher body temperatures (> 37° C) than typically would be found in the upper human respiratory tract (33°C). This is an adaptation that helps serve the virus’ virulence in avian species, which generally have higher resting body temperatures (41°C to 42°C) than humans (37°C).13
Clinical Manifestations in Animals
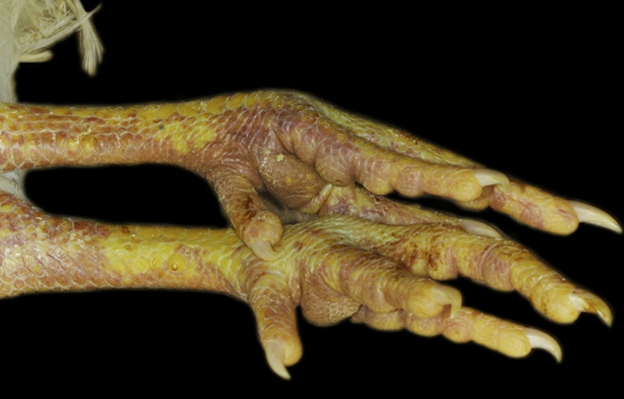
Chickens and poultry infected with HPAI H5 and H7 viruses may suffer from a variety of symptoms. Agricultural workers, veterinarians, and families exposed to commercial or hobby flocks of poultry or other birds should be asked if any of their animals have experienced sudden death, swelling of the eyelids, swelling or discoloration of the wattle, comb, or legs, unexplained changes in egg production or quality, difficulty breathing, nasal discharge, stumbling, twisting of the head or neck, or diarrhea.11 (See Figure 2 and Table 2.) Poultry flocks are highly susceptible to HPAI H5 and H7 viruses, and it is common for an infected flock to be wiped out in several days.
Figure 2. Symptoms of Avian Influenza in Poultry |
 |
Top: Discoloration or hemorrhage of the legs. Bottom: Swelling and purple discoloration of the comb and wattle, along with eyelid edema. United States Department of Agriculture, Animal and Plant Health Inspection Service. Signs of illness. Updated June 17, 2024. https://www.aphis.usda.gov/livestock-poultry-disease/avian/defend-the-flock/signs-illness |
Table 2. Clinical Manifestations of Influenza and HPAI in Animals and Humans | ||
| Manifestations in Humans | Manifestations in Poultry | Manifestations in Dairy Cattle |
|
|
|
HPAI: highly pathogenic avian influenza | ||
Dairy cattle and livestock are more resilient and generally suffer fewer severe symptoms. Again, providers should ask if any dairy cattle that a potential patient has been exposed to have suffered from unexplained changes in milk production or quality, decreases in feeding, decreases in energy, tacky or loose feces, dehydration, or fever.14 (See Table 2.) Most cattle make a complete recovery following infection. Among ducks and other wild birds, HPAI H5 and H7 viruses may not cause any noticeable symptoms at all, although they still are infectious to other avian species.15
Clinical Manifestations in Humans
Typically, influenza A viruses in humans require an incubation of one to four days from the time of exposure to the onset of symptoms.4 Most uncomplicated cases of human influenza A infection are characterized by sudden onset of systemic symptoms, such as fever, myalgias, chills, headaches, malaise, and anorexia.3,4 Respiratory symptoms usually include dry cough, pharyngitis, and nasal congestion and rhinorrhea.3,4
While all of the noted symptoms may be nonspecific symptoms that apply to many upper respiratory viral infections, clinical suspicion for influenza should be raised when systemic symptoms predominate over respiratory symptoms. (See Table 2.) Influenza A infections can become complicated by secondary bacterial infections, most frequently because of bacterial pneumonia caused by Staphylococcus aureus, Streptococcus pneumoniae, Group A Streptococcus, or Haemophilus influenzae.4 The manifestations of such secondary bacterial pneumonias may range from easily treated illnesses from an outpatient standpoint to life-threatening respiratory failure requiring extracorporeal oxygenation support. Additional complications to routine influenza A infection also can include otitis media, myositis, myocarditis, Guillain-Barré syndrome, toxic shock syndrome secondary to staphylococcal or streptococcal secondary infection, and Reye’s syndrome (with concomitant aspirin therapy).3
HPAI infections may lead to similar clinical manifestations, albeit perhaps with more severe features. In prior outbreaks, the spectrum of disease has ranged from simple, uncomplicated influenza-like symptoms to multiorgan failure and death.7,8 While only one death out of 70 cases has been reported in the United States because of HPAI H5N1, globally, the mortality rate has trended closer to 53%, suggesting that strains circulating abroad have been particularly virulent, and the United States experience may be exceptional.8 Other strains of HPAI, such as H7N9, likewise have produced remarkably severe manifestations, with upwards of 70% of patients experiencing severe pulmonary disease, including acute respiratory distress syndrome.8
Of particular note, conjunctivitis and/or subconjunctival hemorrhage often have been features of the H5N1 strain in humans in the United States, frequently without any other respiratory or systemic symptoms.16,17 (See Table 2. An example of conjunctivitis caused by HPAI can be seen at https://bit.ly/4cVKFe9.) H7 influenza subtypes also have been noted to have a particular tropism for eye symptoms.8 Patients with notable contact with poultry and livestock presenting with such ocular symptoms should warrant testing for HPAI.
Diagnosis
Normally, influenza viral testing can be performed with antigen detection or nucleic acid amplification (NAAT) from an appropriate specimen from the respiratory tract.4 For most individuals without relevant high-risk exposures, ordinary seasonal influenza viral testing is encouraged only in symptomatic cases where a meaningful intervention (such as prescribing an antiviral) can be undertaken in a timely fashion. For most outpatient, urgent care, or emergency department settings, such specimens can be obtained from nasopharyngeal swabs.
A variety of commercial tests are offered for rapid antigen testing and nucleic acid amplification, the latter of which can be found as singleplex assays (where the influenza test is performed alone) or as part of multiplex assays (where influenza virus is tested for alongside a panel of other respiratory viruses). For patients experiencing respiratory failure and requiring mechanical ventilation, deeper specimens from endotracheal aspirates or bronchoalveolar lavage may be obtained, especially if initial upper respiratory tract testing was negative.4
Many commercial assays can distinguish influenza A from B or C types, which can be helpful when considering the possibility that a symptomatic case represents an HPAI H5N1 infection — such a case should be positive for influenza A on an assay, but not influenza B or influenza C. This is especially true if the test is capable of distinguishing that the sample is an influenza A virus but not an H1 or H3 subtype.
At the time of this writing, there are no commercially available tests to positively identify H5N1 virus, and most testing must be arranged through local health departments for specific situations. Currently, testing for HPAI H5N1 should not routinely be performed in most individuals.16 However, patients presenting with upper respiratory symptoms or conjunctivitis within 10 days of any high-risk exposures should undergo testing for HPAI H5N1 in coordination with local public health authorities.
High-risk exposures generally include the following: handling, slaughtering, or butchering sick or dead animals known or suspected to be infected with HPAI H5N1; consuming or preparing undercooked meat or unpasteurized dairy of animals known or suspected to be infected with HPAI H5N1; having direct contact with surfaces or objects contaminated with secretions, feces, unpasteurized milk, or carcasses/body parts of animals known or suspected to be infected with HPAI H5N1; visiting a live bird market associated with confirmed or suspected cases of animal or human HPAI H5N1; having recent exposure (being within 6 feet without appropriate respiratory and eye protection) to a known or suspected human case with symptomatic HPAI H5N1 infection; or, lastly, having exposures to HPAI H5N1 in a laboratory setting without appropriate protective equipment.16 (See Table 3.) Testing for H5N1 often will use nasopharyngeal swab specimens for NAAT testing as well as conjunctival swab samples if eye symptoms are prominent or isolated.
Table 3. Situations When Testing for HPAI H5N1 Is Recommended |
If symptomatic with conjunctivitis, fever, myalgias, malaise, headache, cough, nasal congestion, rhinorrhea, and with any of the following exposure(s):
|
HPAI: highly pathogenic avian influenza |
Treatment
At the time of this writing, four drugs currently are routinely recommended for treatment of seasonal influenza infections in the United States. These include the NA inhibitors oseltamivir (Tamiflu), zanamivir (Relenza), and peramivir (Rapivab), as well as the cap-dependent endonuclease inhibitor baloxavir marboxil (Xoflusa).4 Only the mentioned NA inhibitors routinely are used for treatment of influenza A infections. Baloxavir marboxil generally is reserved for influenza B infections.
Antiviral treatment in ordinary influenza A cases generally is recommended in outpatient settings for previously healthy children and adults if therapy can be started within 48 hours of illness onset.4 For children and adults with higher-risk conditions (see Table 1), therapy can be started without regard for the duration of symptoms. For inpatient settings, children and adults with severe or progressive disease or with high-risk symptoms should be given antiviral therapy regardless of duration of symptoms.
Table 1. High-Risk Conditions that Predispose Patients to Severe Outcomes Caused by any Influenza Virus3 |
|
Oseltamivir is an oral/enteral antiviral available for treating patients 2 weeks of age or older or for chemoprophylaxis in patients 3 months of age or older.4 Zanamivir is an inhaled medication that is approved for treatment in patients aged 7 years or older or for chemoprophylaxis in patients 5 years or older. Peramivir is an intravenous drug available for treatment in children 6 months of age or older and does not have a chemoprophylaxis indication.
NA inhibitors generally have been shown to be effective against HPAI strains, particularly H5N1.18 In particular, oseltamivir is recommended for promptly treating cases of confirmed or suspected HPAI H5N1 infection in humans.
Prevention
All suspected cases of avian influenza should be immediately placed in airborne precautions, along with standard and contact precautions.19 This means patients should be placed in an airborne isolation room or transferred to a facility with one available. Until this can be accomplished, the patient should wear a face mask to limit viral droplet spread.
Facility infection prevention specialists should be notified immediately of such cases. Providers should wear National Institute for Occupational Safety and Health (NIOSH)-approved N95 fit-tested respirators, along with disposable gloves, gowns, and eye protection. Only essential personnel should be allowed into the patient’s room or proximity.
Immunization remains an important preventive measure with regard to ordinary seasonal influenza A and B viruses. All individuals aged 6 months and older should receive an annual influenza vaccine.4 Quadrivalent seasonal influenza vaccines typically are composed of antigens from two influenza A strains and two influenza B strains. This practice is intended to protect the vaccine recipient against the strains predicted to predominate in the northern hemisphere that coming season.
Absolute contraindications to the inactivated seasonal influenza vaccine are rare. Anaphylaxis to prior receipt of influenza vaccine is one of the few true absolute contraindications when considering inactivated influenza vaccine.20 History of Guillain-Barré syndrome following inactivated influenza vaccine administration merits caution, but it technically is not a definitive contraindication. Most individuals with egg allergy are capable of tolerating the inactivated influenza vaccine. Febrile illness or other sick symptoms are not contraindications to receiving any seasonal influenza vaccine.
The live influenza vaccine is contraindicated for more groups aside from people who experienced anaphylaxis. Contraindicated patients include people who are immunocompromised, pregnant, have a diagnosis of asthma, have cochlear implants or risk of cerebrospinal fluid leak, or who have recently received oseltamivir or other influenza-targeted antivirals.20
However, it should be emphasized that seasonal influenza vaccines do not necessarily offer any protection against HPAI H5N1 or other avian influenza strains.21 That being said, individuals who could be at risk of contracting avian influenza through agricultural work or contact with sick or dead animals still should receive a seasonal influenza vaccine for two important reasons: first, to prevent illness caused by ordinary circulating influenza, and second, to avoid the rare but potentially catastrophic scenario of contracting both avian influenza and seasonal influenza at the same time, which would help avoid two serious infections at once, or worse, potentially creating a situation where a reassortment of influenza genetic material could create a new strain (or strains) of the virus(es).
At this time, there is no widely available vaccine for HPAI H5N1 — or any other avian influenza strains.21 Previous vaccines have been developed and licensed against prior strains of H5N1, but because of antigenic drift since they were developed, they likely would not confer ideal levels of protection.22 However, multiple candidate vaccines for the currently circulating H5N1 strain in the United States are in various stages of development.
Importantly, newer vaccine technologies, like messenger RNA (mRNA) vaccines, offer a distinct advantage to prior vaccine platforms. Prior influenza vaccines depended on a time-intensive process of growing influenza antigen from chicken eggs, followed by harvesting, purification, and adjuvant addition to create vaccines matched to circulating strains.
However, mRNA vaccines only require the genetic sequence of the target influenza virus to be inserted into the mRNA vehicle. Thereafter, such vaccines may be produced much more rapidly, skipping the antigen-growing step in chicken eggs.22
Moreover, far smaller quantities of vaccine may provide adequate protection, meaning that, during a pandemic, resource-strapped manufacturing facilities potentially can provide more lifesaving doses of vaccine. Animal studies using ferrets have demonstrated that mRNA vaccines can provide good protection against what would otherwise be lethal doses of H5N1 strains — mRNA-vaccinated ferrets survived, and unvaccinated ferrets died from an H5N1 challenge.23
However, until candidate vaccines for HPAI H5N1 and other avian influenza viruses are rolled out more broadly, providers should advise patients and families on practices to reduce the risk of contracting such infections. People should be advised to avoid sick or dead animals, particularly poultry.16 Personal protective equipment (PPE) should be worn whenever working with, in contact with, or in close proximity to (within 6 feet) sick or dead animals, especially poultry or wild birds.
The same precaution applies to working around animal feces or surfaces contaminated with feces or secretions. PPE should include safety goggles, disposable gloves, boots or boot covers, a NIOSH-approved particulate respirator (like an N95 mask), hair cover, and fluid-resistant overalls and aprons.
Poultry, eggs, and beef should be appropriately and fully cooked before consumption.16 Families should be encouraged to choose pasteurized milk to limit potential exposure to HPAI, among other pathogens like Salmonella, Escherichia coli, or Listeria.
If a person is exposed to suspected or confirmed animals with HPAI H1N5 or other avian influenza viruses, providers should inquire if appropriate PPE was worn.16 If the individual was wearing appropriate PPE, they should self-monitor for any symptoms suspicious for avian influenza for the next 10 days. If PPE was not worn, oseltamivir post-exposure chemoprophylaxis may be considered in conjunction with recommendations from public health authorities and infectious diseases specialists.16
Conclusion
HPAI viruses like the H5 and H7 strains could pose a serious threat to human and animal health in the future. Understanding the virology and pathophysiology behind such infections will be important for providers to appropriately educate themselves and the public. Most importantly, providers need to be able to understand which patients are at risk, how to identify such infections, and what to do if they are suspected. Prevention remains essential, and families and the public should be educated on how to reduce the risk of avian influenza infections.
Case Conclusion
Based on the child’s symptoms and risk factors, the provider harbors serious suspicions that this child is infected with avian influenza. The provider dons an N95 respirator with gown, gloves, and eye protection, places a mask on the patient’s face, transfers her to an airborne isolation room, and contacts the facility’s infection preventionists. Then, the provider contacts local public health authorities and infectious diseases specialists.
In consultation with these groups, nasopharyngeal and conjunctival swabs are obtained for NAAT testing and sent to the nearest public health laboratory. In addition, it is decided to initiate a course of oral oseltamivir. The child eventually is released to recuperate at home while her family members are provided a course of chemoprophylactic oseltamivir. She makes a full recovery. Public health investigators determine that the free-range chickens on her family farm may have been the source of her infection, and the animals are culled and removed.
Trahern Jones, MD, is Assistant Professor, Pediatric Infectious Diseases, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City.
References
- Xie R, Edwards KM, Wille M, et al. The episodic resurgence of highly pathogenic avian influenza H5 virus. Nature. 2023;622(7984):810-817.
- Centers for Disease Control and Prevention. H5 bird flu: Current situation. Last updated April 15, 2025. https://www.cdc.gov/bird-flu/situation-summary/index.html
- Treanor JJ. Influenza viruses, including avian influenza and swine influenza. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier;2020:2143-2168.
- American Academy of Pediatrics. Influenza. In: Kimberlin DW, Banerjee R, Barnett ED, Lynfield R, Sawyer MH, eds. Red Book: 2024-2027 Report of the Committee on Infectious Diseases. American Academy of Pediatrics;2024:511-522.
- Hall E. Chapter 12: Influenza. In: Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hall E, Wodi AP, Hamborsky J, et al, eds. 14th ed. Public Health Foundation;2021: https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-12-influenza.html
- Centers for Disease Control and Prevention. Preliminary estimated flu disease burden 2023-2024 flu season. Updated Nov. 20, 2024. https://www.cdc.gov/flu-burden/php/data-vis/2023-2024.html
- Yuen KY, Chan PK, Peiris M, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351(9101):467-471.
- McCullers JA. Influenza Viruses. In: Cherry JD, Kaplan SL, Harrison GJ, et al, eds. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. 9th ed. Elsevier;2025:1811-1828.
- Centers for Disease Control and Prevention. Past reported global human cases with highly pathogenic avian influenza A (H5N1) (HPAI H5N1) by country, 1997-2025. Updated March 25, 2025. https://www.cdc.gov/bird-flu/php/avian-flu-summary/chart-epi-curve-ah5n1.html
- Centers for Disease Control and Prevention. 2020-2024 highlights in the history of avian influenza (bird flu) timeline. Published April 30, 2024. https://www.cdc.gov/bird-flu/avian-timeline/2020s.html
- United States Department of Agriculture, Animal and Plant Health Inspection Service. Avian influenza. Updated March 19, 2025. https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza
- Centers for Disease Control and Prevention. Avian influenza in birds: Causes and how it spreads. Updated March 26, 2025. https://www.cdc.gov/bird-flu/virus-transmission/avian-in-birds.html
- Li YT, Linster M, Mendenhall IH, et al. Avian influenza viruses in humans: Lessons from past outbreaks. Br Med Bull. 2019;132(1):81-95.
- United States Department of Agriculture, Animal and Plant Health Inspection Service. Avian Influenza. Updated Dec. 20, 2024. https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-livestock
- Centers for Disease Control and Prevention. Current situation: Bird flu in wild birds. Updated May 2, 2024. https://www.cdc.gov/bird-flu/situation-summary/wildbirds.html
- Centers for Disease Control and Prevention. Highly pathogenic avian influenza A (H5N1) virus: Interim recommendations for prevention, monitoring, and public health investigations. Published Dec. 26, 2024. https://www.cdc.gov/bird-flu/prevention/hpai-interim-recommendations.html
- Garg S, Reinhart K, Couture A, et al. Highly pathogenic avian influenza A (H5N1) virus infections in humans. N Engl J Med. 2025;392(9):843-854.
- Centers for Disease Control and Prevention. CDC A (H5N1) bird flu response update March 19, 2025. Published March 19, 2025. https://www.cdc.gov/bird-flu/spotlights/h5n1-response-03192025.html
- Centers for Disease Control and Prevention. Interim guidance for infection control within healthcare settings when caring for confirmed cases, probable cases, and cases under investigation for infection with novel influenza A viruses associated with severe disease. Updated March 9, 2025. https://www.cdc.gov/bird-flu/hcp/novel-flu-infection-control/
- Centers for Disease Control and Prevention. Influenza (flu): ACIP recommendations. Updated Sept. 17, 2024. https://www.cdc.gov/flu/hcp/acip/?CDC_AAref_Val=https://www.cdc.gov/flu/professionals/acip/summary/summary-recommendations.htm
- Centers for Disease Control and Prevention. Prevention and antiviral treatment of avian influenza A viruses in people. Updated July 19, 2024. https://www.cdc.gov/bird-flu/prevention/index.html
- Barron M. Avian influenza (H5N1) vaccines: What’s the status? American Society for Microbiology. Published March 4, 2025. https://asm.org/articles/2025/march/avian-influenza-h5n1-vaccines-what-status
- Hatta M, Hatta Y, Choi A, et al. An influenza mRNA vaccine protects ferrets from lethal infection with highly pathogenic avian influenza A (H5N1) virus. Sci Transl Med. 2024;16(778):eads1273.
- United States Department of Agriculture, Animal and Plant Health Inspection Service. Signs of illness. Updated June 17, 2024. https://www.aphis.usda.gov/livestock-poultry-disease/avian/defend-the-flock/signs-illness