Pediatric Airway Management: The Basics
June 1, 2025
By Alexandra Armato, MD, and Daniel Migliaccio, MD, FPD, FAAEM
Executive Summary
- Neonates can develop significant respiratory distress and feeding problems with nasal obstruction, which may be congenital (as seen in choanal atresia) or acquired (as in nasal congestion with upper respiratory infections).
- The benefits of high-flow nasal cannula (relative to nasal cannula) include decreased nasopharyngeal resistance, washout of dead space, reduction of inflow of ambient air, and increase in airway pressure. This method commonly is used in patients with bronchiolitis, status asthmaticus, and mildly to moderately increased work of breathing.
- Two-rescuer bag-mask ventilation (BMV) may be necessary in the settings of inadequate inflation pressure caused by a poor seal between the ventilation mask and the face, increased airway resistance (as seen in pneumonia), poor lung compliance (as seen in pneumonia or pulmonary edema), and/or cervical spine motion restriction (as seen in suspected cervical spine injury). The two-rescuer BMV technique has been proven to provide greater peak pressures and mean tidal volumes per weight compared to one-rescuer BMV. Two-rescuer BMV is considered standard of care in situations with enough personnel available.
- To determine the appropriately sized endotracheal tube (ETT) in children older than 1 year of age, use the equation (age / 4) + 4 for uncuffed ETTs and the equation (age / 4) + 3.5 for cuffed ETTs. Point-of-care ultrasound also has been proven to be useful for determining the appropriately sized ETT. In fact, this method has been shown to be more accurate than formula-based estimations. To use bedside ultrasound, the minimal transverse diameter of the supraglottic airway at the level of the cricoid cartilage may be measured by placing the linear probe on the anterior midline of the neck.
- For patients with hypotension other than septic shock, etomidate is the sedative agent of choice because of its preservation of hemodynamic stability. For patients with hypotension caused by septic shock, ketamine should be the first-line sedative agent, given etomidate’s potential for adrenal suppression. For patients with increased intracranial pressure, etomidate should be the sedative of choice; propofol also is an appropriate second-line sedative agent. For hypotensive patients with a head injury, the sedative agent of choice should be etomidate. For status asthmaticus, the sedative agent of choice should be ketamine because of its bronchodilator effects, although etomidate also is an appropriate choice. For hemodynamically stable patients with status epilepticus, midazolam or propofol may be used.
- Absolute contraindications to succinylcholine include neuromuscular disease, such as Becker or Duchenne muscular dystrophy and cerebral palsy with paralysis, severe burns, crush injuries and severe traumas, rhabdomyolysis, history of malignant hyperthermia in patient or relatives, and hyperkalemia. Relative contraindications include increased intracranial pressure, increased intraocular pressure, and known pseudocholinesterase deficiency.
Introduction
A child with severe respiratory distress often is a stressful experience, even to the most experienced providers. Pediatric airways present unique challenges relative to adults. This article will discuss pediatric airway topics, including differences between adult and pediatric airways, bag-mask ventilation (BMV) and other noninvasive forms of ventilation, rapid sequence intubation, and the approach to a difficult pediatric airway.
Anatomic Considerations
There are multiple anatomic differences between pediatric and adult airways that change the mechanics of intubation and ventilation. These differences gradually become less noticeable as the child grows, with more comparable proportions — albeit smaller size — than an adult airway around 8 years of age. Understanding these differences in infancy and early childhood is key to optimizing the technique of positioning and intubating a pediatric airway.1,2
Head/Occiput
Compared to adults, infants and young children have a relatively larger occiput relative to the rest of their bodies. The larger occiput results in passive flexion of the cervical spine while supine, leading to an increased potential for airway occlusion. This potential implication can be overcome by maintaining the sniffing position, which extends the neck and aligns the pharyngeal and tracheal axes.1,2 Placing a rolled towel underneath the shoulders in infants or underneath the occiput in children older than 2 years of age may help to maintain this position and optimize the laryngoscopic view.3
Nasopharynx and Hypopharynx
In neonates, the nasopharynx and hypopharynx have a close proximity to each other because of the low-lying posterior soft palate and enlarged epiglottis. This anatomic difference physiologically allows the neonate to simultaneously feed and breathe while reducing the risk of aspiration. The distance between the nasopharynx and hypopharynx gradually increases throughout the first year of life as the infant grows.1 Because of constraints with mouth breathing secondary to this normal neonatal anatomy, neonates often are described as obligate nasal breathers until around 6 months of age, although this population is able to breathe through the mouth both spontaneously and with nasal obstruction. Neonates can develop significant respiratory distress and feeding problems with nasal obstruction, which may be congenital (as seen in choanal atresia) or acquired (as in nasal congestion with upper respiratory infections).2
Tongue, Adenoids, and Tonsils
In infants and children, the tongue is relatively larger within the oral cavity when compared to adults. This anatomic difference results in the tongue falling backward against the hypopharynx, leading to increased potential for airway obstruction with decreased level of consciousness or paralysis. Additionally, the adenoids and tonsils are larger within the oral cavity in infants and young children relative to adults, which also contributes to airway obstruction.1,2 Airway maneuvers to overcome this implication include the jaw thrust or chin lift. Oral or nasopharyngeal airways (NPAs) may help to temporarily reduce airway obstruction and assist with ventilation.1
Epiglottis
The glottic opening, epiglottis, and aryepiglottic folds are softer, more prominent, and more mobile in an infant or young child than in an adult. This “floppy” redundant tissue can obscure the view of the cords. For this reason, when intubating an infant or young child, the laryngoscopist should attempt to lift the epiglottis directly to visualize the cords, which often is more easily achieved using a straight blade.1,2
Larynx
In infants and children, the larynx is superior with more anterior vocal cords relative to the larynx of adults. Therefore, hyperextension of the neck in positioning the child prior to intubation may worsen obstruction of the upper airway and make visualization of the cords more difficult. Using a rolled towel underneath the shoulders in infants or under the occiput in children older than 2 years of age also can help to overcome this difficulty by better aligning the airway axes so that the tragus is aligned with the clavicle. Video laryngoscopy (relative to direct laryngoscopy) also has been shown to improve the view of the glottis.1,2
Trachea
The smaller diameter of the airway of infants and children increases susceptibility to obstruction by secretions, blood, emesis, and foreign bodies. Therefore, suctioning is paramount in clearing and maintaining the airway in the setting of respiratory distress.2 A child’s trachea is narrowest at the cricoid ring, which is a common site of mucosal swelling in croup.1,2 This principle is important when considering cuff insufflation pressures, which should be monitored carefully.1
Additionally, any reduction in diameter to this already narrow aspect of the trachea increases airway resistance, especially when airflow becomes turbulent. Swollen epiglottic, glottic, and subglottic tissues create a dynamic obstruction in the setting of increased airway resistance, which can progress to complete obstruction and respiratory arrest.2 Keeping an infant or child with partial airway obstruction calm will help to slow this progression. Airway obstruction often worsens with negative airway pressure during inspiration, so children with airway edema and obstruction usually respond well to positive pressure ventilation, such as bag-valve mask or continuous positive airway pressure.1
Trachea
The size of the cricothyroid membrane in children younger than 10 years old is too small for a clinician to appreciate the proper landmarks for surgical cricothyrotomy. Therefore, an open cricothyrotomy is contraindicated in children younger than 10 years old; percutaneous needle cricothyrotomy is the preferred invasive subglottic airway method.1,4
Physiologic Considerations
In addition to anatomic differences, infants and children also have key important physiologic differences to consider in ventilation and oxygenation during pediatric resuscitation.5
Increased Metabolic Rate and Oxygen Consumption
Infants and children have a faster metabolic rate than adults, which means that oxygen consumption is increased. Additionally, children have a relatively small lung volume and functional residual capacity, so children often compensate for any insult to oxygenation or ventilation by increasing their respiratory rate.5 Children also have a reduced functional residual capacity relative to adults, which leads to a shorter length of safe apnea time after neuromuscular blockade, even with preoxygenation.1,5 These physiologic considerations result in increased vulnerability to rapid desaturation when oxygenation or ventilation is reduced. Because of the potential for rapid desaturation and decompensation, the child should undergo apneic ventilation and BMV prior to an intubation attempt during the apneic period after paralytics are administered.6,7
Gastric Insufflation
Children and infants have a smaller functional residual capacity than adults, which allows their lungs to become overinflated more quickly. This physiologic difference, along with increased upper airway collapsibility and resistance relative to adults, can result in more rapid gastric distention due to insufflation of the stomach during bag-valve mask ventilation.8 Gastric insufflation and distention can further reduce the functional residual capacity, tidal volume, and ventilation and compromise the respiratory status. Proper bag-valve mask technique is key in minimizing gastric insufflation during BMV. The rescuer should avoid excessive peak inspiratory pressures by ventilating slowly and watching chest rise.9
Extracellular Fluid Space
Children have a proportionally larger extracellular fluid compartment than adults, resulting in a quicker onset and shorter duration of action of drugs. Therefore, children may require higher doses of drugs per kilogram than adults.10
Noninvasive Airway Maneuvers
To help a child with signs of anatomic airway obstruction, optimize positioning using the sniffing position. Manual maneuvers also may be performed to help open and maintain the airway, including the head tilt-chin left maneuver and the jaw thrust maneuver. These maneuvers are particularly helpful in patients in the setting of temporary reversible sedation, such as is used during procedures.11
The preferred method is the head tilt-chin lift maneuver, except when cervical spine injury is suspected. To perform this maneuver, place the fingers of one hand underneath the mandible and lift upward to move the chin anteriorly, and use the thumb to lightly depress the lower lip to keep the mouth open. Place the other hand on the child’s forehead to gently tilt the head in a neutral position.9 Avoid pressure to the soft tissues underneath the chin or hyperextending the neck, both of which may worsen obstruction.12
In patients with concern for cervical spine injury, the jaw thrust maneuver should be attempted first while maintaining in-line stabilization. The rescuer should hook the thumbs behind the angles of the mandible and move the mandible forward. If this maneuver does not work, the rescuer then may gently attempt the head tilt-chin lift maneuver while maintaining in-line cervical spine stabilization.13
Noninvasive Oxygenation and Ventilation
Multiple forms of noninvasive ventilation and oxygenation exist that may be used in patients to improve oxygen levels before more invasive measures, such as intubation.
Standard Nasal Cannula
Standard nasal cannula may be used in patients who are hypoxic to provide higher levels of inspired oxygen. Common settings in children are 0.5 L/min to 4 L/min. Oxygen flow should be titrated to achieve an oxygen saturation of 96% to 100% with the lowest setting possible.14 This method also is used during intubation for apneic oxygenation as described later; flow rates used for apneic oxygenation are higher at 5 L/min for infants and 10 L/min to 15 L/min for older children. Oxygenation through a nasal cannula does not provide ventilatory support, but rather supports oxygenation.7
Heliox
Another noninvasive ventilation method to provide oxygenation is heliox. Heliox is a mixture of oxygen and helium that uses the low density of helium to improve gas flow through high resistance airways. In scenarios of high airway resistance, heliox is used to provide a higher flow rate and change the turbulent flow pattern to a more laminar flow pattern with improved diffusion.15,16
The method of administration of heliox is through a specialized mask similar to a non-rebreather. This gas mixture is described in concentrations of helium to oxygen. The typical helium-to-oxygen concentrations that are used in the intensive care unit are 80:20, 70:30, and 60:40. With higher concentrations of helium in the gas mixture, the flow of air becomes less turbulent and is delivered at a higher rate. However, many patients require a higher oxygen concentration to maintain sufficient oxygen saturation. Studies have shown that, for heliox to be effective, the helium concentration ideally must be greater than or equal to 60%, so patients who are not able to maintain SpO2 > 92% with 40% oxygen do not benefit significantly from heliox.16
Heliox is especially useful in severe asthma exacerbations and status asthmaticus, given the pathophysiology of restricted gas flow through narrow airways. The administration of this gas mixture has been shown to improve pulsus paradoxus and peak expiratory flow in non-intubated patients with severe asthma. Research suggests that heliox is more effective for the more severe asthma exacerbations and is most beneficial when used early in the disease course within the first 24 hours of onset. Heliox may be used as a temporizing measure to prevent intubation in sick asthma exacerbations.17
Heliox also may be used in young children in the setting of bronchiolitis with severe respiratory distress. Studies have shown a significant reduction of clinical scores evaluating respiratory distress in non-intubated infants with severe respiratory distress secondary to bronchiolitis. However, heliox has not been shown to reduce the need for intubation and mechanical ventilation in such cases.18
High-Flow Nasal Cannula
High-flow nasal cannula is a form of noninvasive ventilation that delivers heated and humidified oxygen into the airway using two large bore nasal prongs. The benefits of high-flow nasal cannula (relative to nasal cannula) include decreased nasopharyngeal resistance, washout of dead space, reduction of inflow of ambient air, and increase in airway pressure.19 This method commonly is used in patients with bronchiolitis, status asthmaticus, and mildly to moderately increased work of breathing.20 High-flow nasal cannula has been shown to increase end-expiratory lung volume and improve respiratory rate and oxygen saturation. This method also may be used for apneic oxygenation during the apneic period of rapid sequence intubation. The setting most frequently used is 1 L/kg/min to 2 L/kg/min. Higher flow rates can create positive airway pressure to improve ventilation and reduce the need for subsequent intubation in certain cases.21,22
Positive Pressure Ventilation
Continuous positive airway pressure (CPAP) is a form of noninvasive positive pressure ventilation that delivers one constant airway pressure throughout the respiratory cycle, equivalent to providing positive end expiratory pressure (PEEP) without any other support. CPAP can provide sufficient ventilation in patients with acute hypoxic respiratory failure, upper airway obstruction, bronchiolitis, status asthmaticus, and moderately to severely increased work of breathing.23 The rate for CPAP typically is 5 cm H2O to 8 cm H2O.22,24
Bilevel positive airway pressure (BiPAP) is a form of noninvasive positive pressure ventilation that cycles between delivery of two pressure levels — the inspiratory positive airway pressure (IPAP) during inspiration and the expiratory positive airway pressure (EPAP) during the expiratory phase. EPAP typically is delivered at a range of 4 cm H2O to 8 cm H2O. Meanwhile, during the inspiratory phase, an additional inspiratory pressure support is delivered on top of the baseline EPAP pressure. These two pressures combined are known as the IPAP, with a typical range from 8 cm H2O to 15 cm H2O.24 BiPAP provides ventilatory assistance to patients with asthma, neuromuscular disorders, bronchiolitis, acute hypoxic respiratory failure, and acute hypercapnic respiratory failure.22,25
These forms of ventilation are most effective when initiated early in the disease process. The FiO2 rates should be kept as low as possible so that the provider can know the true oxygen requirement of the patient and anticipate next steps if respiratory failure is unable to be controlled with BiPAP. Positive airway pressure with CPAP and BiPAP increases the risk for aspiration and should be avoided in patients who are vomiting or not protecting their airway.22,24
Bag-Mask Ventilation
BMV is an important skill in pediatric resuscitation that can be applied in many clinical scenarios. In cases in which rapid recovery is anticipated, such as apnea/hypopnea with deep sedation, BMV often is sufficient to support oxygenation without the need for more invasive measures. Additionally, BMV is paramount in preventing desaturation events during the apneic period following induction for intubation and between intubation attempts. BMV also is used during cardiac arrest prior to establishment of an advanced airway.9
Equipment
Effective BMV requires proper equipment with correctly sized bags and masks to ensure an airtight seal and provide appropriate ventilation. The correct size mask is the smallest mask that completely covers the mouth and nose without covering the eyes or overlapping the chin.9 To ensure adequate ventilation with chest rise, a ventilation bag with a minimum volume of 500 mL should be used in infants and younger children, while a ventilation bag with a minimum volume of 1,000 mL should be used in older children and adolescents.26
Manometers may be used to monitor ventilatory pressures during BMV. Attempting to keep inspiratory pressures as low as possible will limit gastric sufflation.27 PEEP valves may be used on many bag-valve mask systems to improve oxygenation in patients with hypoxemia.9
Most pediatric ventilation bags employ a safety pop-off valve that opens at a preset peak pressure (usually 35 cm H2O to 45 cm H2O) to reduce the risk of barotrauma. However, certain clinical scenarios require higher levels of peak inspiratory pressures to provide adequate tidal volumes and ventilation in pathologies with high airway resistance of low lung compliance, such as asthma or airway obstruction. The pop-off valve may be disabled or occluded manually in these cases. However, the clinician must use care to avoid barotrauma in these cases.28
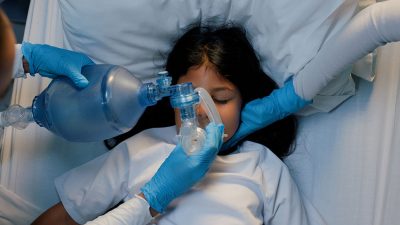
Figure 1 depicts ventilation masks designed for children and infants.
Figure 1. Ventilation Masks |
 |
Masks designed for children (left) and infants (right). Courtesy of Dr. Alexandra Armato and Dr. Daniel Migliaccio. |
Positioning and Technique
First, the patency of the child’s airway should be optimized by flexing the neck such that the external auditory canal is anterior to the shoulder and extending the head on the neck such that the nose and mouth are pointing to the ceiling.9 This position may be best accomplished by placing a roll underneath the occiput in a child or underneath the shoulders in an infant.3 However, in the setting of suspected cervical spine injury, in-line manual stabilization must be maintained, and airway management should be accomplished with minimal mobilization of the neck.29
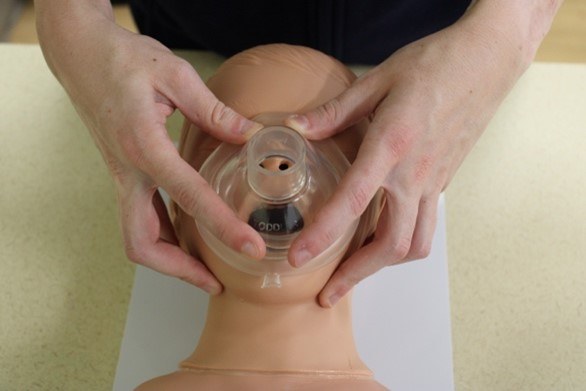
The hand positioning for the ventilation mask is called the E-C clamp technique. The ventilation mask should be positioned over the child’s face with the narrowest portion of the mask over the bridge of the nose.9
In single-rescuer ventilation, the third through fifth fingers of one hand are spread along the mandible, with the fifth finger behind the angle of the mandible forming the shape of an E. The fingers should work to lift the jaw and pull the face into the ventilation mask. The rescuer’s thumb and the forefinger of the same hand should form a C shape onto the mask to press the mask into the child’s face and form a seal between the mask and face. Breaths are provided by using the other hand to squeeze the ventilation bag to the point of visible chest rise.9
In two-rescuer ventilation, one rescuer uses both hands to seal the mask in the same E-C configuration while another rescuer squeezes the ventilation bag.9 An additional two-rescuer technique is the V-E technique, in which one rescuer uses the thenar eminences to press the mask into the face while using the second through fifth fingers to pull the mandible and face into the mask.30
Two-rescuer BMV may be necessary in the settings of inadequate inflation pressure caused by a poor seal between the ventilation mask and the face, increased airway resistance (as is seen in pneumonia), poor lung compliance (as seen in pneumonia or pulmonary edema), and/or cervical spine motion restriction (as seen in suspected cervical spine injury). The two-rescuer BMV has been proven to provide greater peak pressures and mean tidal volumes per weight compared to one-rescuer BMV. Two-rescuer BMV is considered standard of care in situations with enough personnel available.31
In all techniques of BMV, the focus should be pulling the airway up into the mask rather than pushing the mask down into the face. Care should be taken to apply pressure to the bony aspect of the mandible rather than to the submandibular tissue because pressing on these soft tissues can lead to airway obstruction by the tongue.9
Figure 2 depicts the hand positioning for both the single- and two-rescuer E-C techniques.
Figure 2. E-C Techniques |
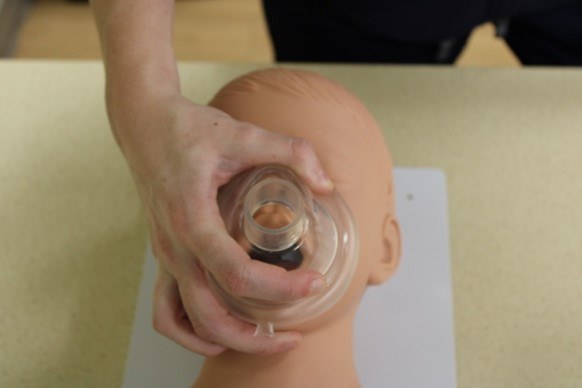 |
Top: The two-rescuer E-C technique Bottom: The single-rescuer E-C technique Courtesy of Dr. Alexandra Armato and Dr. Daniel Migliaccio. |
Respiratory Rate and Volume
The goal ventilation rate of a child requiring ventilation without compressions or with an advanced airway in place is 20 breaths per minute to 30 breaths per minute. During cardiopulmonary resuscitation without an advanced airway, a ratio of two breaths to 30 compressions should be employed with a single rescuer or two breaths to 15 compressions for multiple rescuers. Deliver each breath over one second.32 Regarding tidal volume, the rescuer should squeeze the bag only to the point of chest rise to avoid barotrauma and gastric insufflation.9
Airway Adjuncts
If the airway is not able to be maintained through positioning techniques, more invasive maneuvers with airway adjuncts should be considered.
NPAs, also called “nasal trumpets,” can help remove anatomic obstruction in a semiconscious child with an intact gag reflex by displacing the tongue from the posterior pharynx and soft palate. NPAs are used in the awake patient with a strong gag reflex or in patients with limited oral mobility or aperture (as seen in trismus or angioedema). NPAs are contraindicated in patients with basilar skull fractures and significant facial trauma.33 The proper size of a nasopharyngeal airway is determined by measuring the distance from the tip of the nose to the ear tragus.34 To place, lubricate the NPA with a water-soluble substance. A topical vasoconstrictor also may be applied to each nostril. Gently insert the NPA into a nostril in a posterior direction with the bevel facing the nasal septum. If resistance is met, withdraw and reattempt insertion in the other nostril.9,33
In an unconscious child without a gag reflex, an oropharyngeal airway may be used to assist with ventilation by pushing the tongue away from the posterior pharynx and open the airway. (See Figure 3.) Oropharyngeal airways are especially helpful in patients with poor pharyngeal tone or a large tongue leading to anatomical upper airway obstruction. Oropharyngeal airways should not be used in awake patients because they may trigger the gag reflex and lead to vomiting and aspiration. The proper size of an oropharyngeal airway is determined by measuring the distance from the corner of the mouth to the angle of the mandible. To place, depress the tongue using a tongue blade, then gently insert the oropharyngeal airway along the curve of the mouth and pharynx.35
Figure 3. Sizing Oropharyngeal Airways |

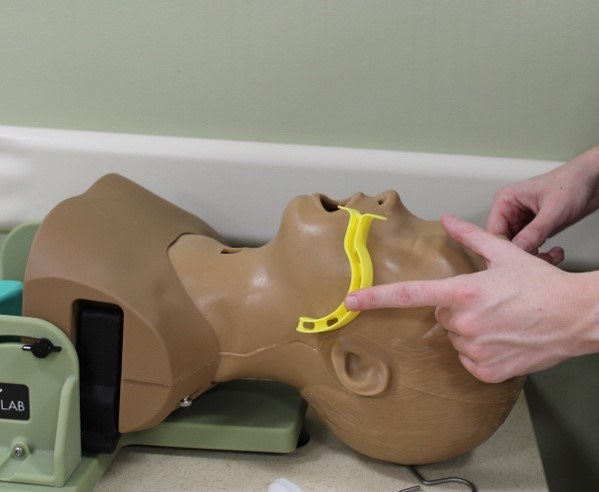 |
Top: Oropharyngeal airways Bottom: Appropriate sizing of an oropharyngeal airway Courtesy of Dr. Alexandra Armato and Dr. Daniel Migliaccio. |
Pitfalls and Common Errors
In the setting of difficult BMV, the initial step to troubleshoot is to reposition the patient. Check the seal of the mask and consider changing the hand grip on the mask or size of the mask. As discussed, adjunct airways may be placed to relieve anatomic airway obstruction. Inspect the airway for foreign bodies obstructing the airway. Avoid excessive ventilation, which can result in gastric insufflation and in turn lead to impaired ventilation and reduced cardiac output.9
Another common cause for difficulty in ventilating pediatric airways with a bag-valve mask is airway obstruction by secretions or airway debris. Suctioning can help to clear debris and secretions to improve efficacy of ventilation. Clinicians should use a large-bore soft catheter to rapidly and effectively suction secretions and debris in children undergoing BMV. Prolonged suctioning longer than 15 to 20 seconds should be avoided because of the risk of hypoxia and bradycardia.36
A possible pitfall with BMV in children with certain congenital conditions is difficulty with mask fit for faces of children with syndromes that alter head and neck anatomy. Clinicians should anticipate difficulty in BMV of children with retrognathia, micrognathia, and patients with short necks or limited neck mobility.37 If the discussed measures and adjuncts are not sufficient to help oxygenate and ventilate the patient with BMV, the provider should prepare for endotracheal intubation.
Endotracheal Intubation: Rapid Sequence Intubation
Rapid sequence intubation (RSI) is a systematic form of intubation that involves administration of a sedative and a paralytic medication in rapid succession to facilitate a safe emergency endotracheal intubation. RSI provides optimal conditions for emergency intubation because of its higher safety profile and higher first-pass success rates than without sedation and paralysis, especially in patients with decreased levels of consciousness, with diminished airway protection, and/or with a high risk of aspiration.38
The steps of RSI are preparation, positioning, preoxygenation, pretreatment, paralysis/induction, placement of the endotracheal tube (ETT) with confirmation of proper positioning, and post-intubation management.39 (See Table 1.)
Table 1. Steps of Rapid Sequence Intubation |
Preparation and Planning
Preoxygenation
Pretreatment
Paralysis and Induction
Protection and Positioning
Placement with Proof
Post-Intubation Management
|
Preparation
Preparation involves ensuring that all equipment is at the bedside and ready for use, all personnel required for the intubation are present, and medications are prepared.
A challenge that becomes particularly important in the pediatric population is selection of appropriately sized equipment and administration of proper weight-based doses of medications. Many equations exist to assist clinicians in selecting the proper equipment. The Broselow tape is a bedside measuring tape that uses the child’s height to determine appropriate sizing and doses of medications and can be a useful resource for pediatric intubations.40
Equipment that should be at the bedside includes a suction device, a bag-valve mask device connected to an oxygen source, the ETT with a stylet, a blade or video laryngoscopy supplies, and an end tidal carbon dioxide detector. The ventilator should be available and ready to attach to the ETT once placed. A backup airway kit, including a bougie, laryngeal mask airways, and equipment for a surgical airway, should be readily available in case the airway proceduralist encounters difficulty with intubation. A resuscitation cart also should be readily available in case the patient loses pulses during intubation. Medications of the appropriate dose should be drawn and prepared to be administered.11
Medications
Prior to intubation, ensure that medications of the proper dose for the child’s size are drawn and prepared to be administered. Medications that should be readily available are the RSI drugs and any premedication drugs that the proceduralist plans to be administered. Selection and dose of pretreatment drugs and RSI drugs will be discussed in further detail.
Endotracheal Tubes
An ETT of the correct size with a stylet in place should be prepared and at the bedside prior to initiation of RSI. The ETT should be prepared by molding the stylet into appropriate position with a bend of no more than 35 degrees, described as a “hockey stick” bend. If using video laryngoscopy with a hyperangulated blade, the proceduralist should use a rigid stylet instead of the flexible stylet.41 The cuff should be checked for inflation, checked for defects, and then deflated.42
To determine the appropriately sized ETT in children older than 1 year of age, use the equation (age / 4) + 4 for uncuffed ETTs and the equation (age / 4) + 3.5 for cuffed ETTs.43 Point-of-care ultrasound also has been proven to be useful for determining the appropriately sized ETT. In fact, this method has been shown to be more accurate than formula-based estimations. To use bedside ultrasound, the minimal transverse diameter of the supraglottic airway at the level of the cricoid cartilage may be measured by placing the linear probe on the anterior midline of the neck.44
Cuffed ETTs generally are recommended at the size of 3.5 internal diameter or more. Uncuffed tubes typically result in significant air leaks and are at a higher risk of being misplaced, thus requiring replacement. Additionally, uncuffed tubes often are unable to provide the high ventilation pressures required by many patients intubated in the emergency department.42
Laryngoscope Blades
In children, the straight Miller blade is preferred because of the anatomy of the pediatric airway. Using the straight blade allows the laryngoscopist to lift the relatively larger epiglottis directly and more easily displace the larger tongue. In older children, the curved MacIntosh blades may be used to indirectly lift the epiglottis.45
The proper size of the laryngoscope blade should be determined by placing the blade handle at the central incisors. The end of the blade should be within 1 cm of the angle of the mandible.46 For premature babies, a size 00 blade should be used. For newborns, a size 0 blade should be used. For infants, a size 1 blade generally should be used. A common mnemonic used by physicians to estimate the size of the laryngoscope blade is “2 blade starting at 2 years old, 3 blade in third grade.” If between sizes, the proper laryngoscopic view is more easily obtained using a blade that is too long rather than too short because the blade must be able to reach the supraglottic area.47
Video laryngoscopy has become more frequently used in pediatric intubations, and many providers have moved to use video laryngoscopy as the first-line technique for intubation. Studies have shown that first-pass success rates are higher than with direct laryngoscopy. However, the size of the video laryngoscopy blade is bulkier than the direct laryngoscopy blade, which may complicate maneuvering the ETT into the correct position in between the vocal cords despite an optimal laryngoscopic view. The sizing of video laryngoscope blades is similar to the sizing of direct laryngoscopy blades.41,48 (See Figure 4.)
Figure 4. Pediatric Airway Equipment |
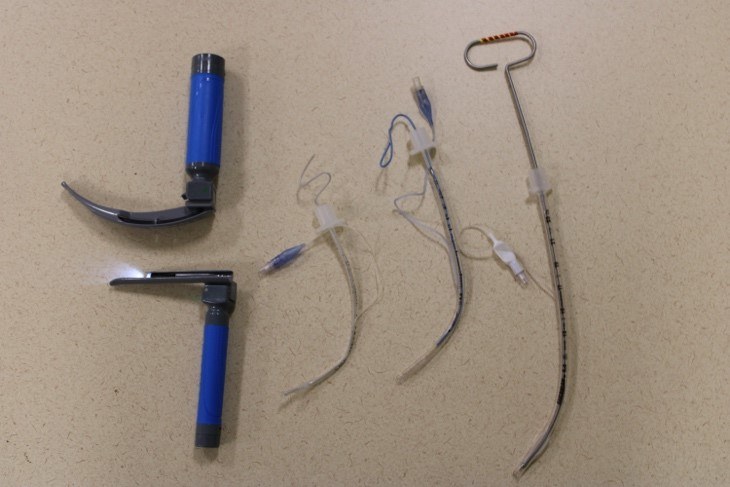 |
The blade depicted at the top left is the curved MacIntosh blade. The blade depicted at the bottom left is the straight Miller blade. To the right are three different sized tubes with stylets of corresponding sizes. Courtesy of Dr. Alexandra Armato and Dr. Daniel Migliaccio. |
Difficult Airway Adjuncts
The provider always should have a backup plan in the setting of failed intubation with a difficult airway. Supraglottic airway devices and the bougie introducer should be readily available.11 The selection of alternative airway techniques is discussed in further detail later in this article. (See Table 2.)
Table 2. Proper Ventilation Equipment Sizing | ||||||
| Age | Weight | BVM | Oral Airway | Blade | ETT | NGT |
Newborn | 3.5 kg | Infant | Infant 50 mm | #0-1 | 2.5-3.5 | 5-8 Fr |
3 months | 6 kg | Infant | Small 60 mm | #1 | 3.5-4.0 | 5-8 Fr |
6 months | 8 kg | Child | Small 60 mm | #1 | 3.5-4.0 | 8-10 Fr |
1 year | 10 kg | Child | Small 60 mm | #1 | 4.0-4.5 | 8-10 Fr |
2 years | 12 kg | Child | Small 70 mm | #2 | 4.0-4.5 | 10 Fr |
3 years | 15 kg | Child | Small 70 mm | #2 | 4.5-5.0 | 10 Fr |
4 years | 17 kg | Child | Med 80 mm | #2 | 4.5-5.0 | 10-12 Fr |
6 years | 20 kg | Child | Med 90 mm | #2 | 5.0-5.5 | 12-14 Fr |
8 years | 25 kg | Child/adult | Med 90 mm | #2-3 | 5.5-6.5 | 14 Fr |
12 years | 40 kg | Adult | Large 100 mm | #3 | 6.0-7.0 | 14-18 Fr |
14 years | 50 kg | Adult | Large 100 mm | #3 | 7.0-8.0 | 14-18 Fr |
BVM: bag-valve mask; ETT: endotracheal tube; NGT: nasogastric tube | ||||||
Clinicians should ensure that the patient is connected to appropriate vital sign monitoring. Vital signs should be continuously monitored during RSI, including cardiac monitoring, pulse oximetry, blood pressure measurements, and end tidal carbon dioxide monitoring. The patient should have at least one (but preferably two) functional points of access, preferably in the form of an intravenous line, although an intraosseous line also suffices in emergent scenarios. Many drugs also are possible to administer via the intramuscular route, but this form of administration should be used only if practitioners are unable to obtain an intravenous or intraosseous line.48
The airway team should be a minimum of three people, including the airway proceduralist, the airway assistant, and a drug administrator. Respiratory therapy specialists and emergency department pharmacists should be called to the bedside if available.48
A common mnemonic used among providers to prepare for intubation is “SOAP ME” — suction, oxygen, airway, position, monitoring and medications, everything else (to include backup airway devices and resuscitation drugs).
If able, practitioners should review key aspects of the child’s medical history and history of present illness to choose the appropriate RSI medications and to anticipate any difficulty with ventilation or intubation. Important information includes any previous difficulty with intubation, previous adverse effects from anesthesia, and any allergies to medications. Certain medical conditions may be a contraindication to certain RSI drugs, as will be discussed later. In children with a history of asthma, laryngoscopy may precipitate bronchospasm. A history of obstructive sleep apnea or noisy breathing with sleep may suggest anatomic airway obstruction, such as enlarged tonsils or tongue, which may make BMV or laryngoscopy more difficult.49
Physical examination findings also should be used to anticipate any difficulties during RSI and to determine appropriate RSI medications. Various physical examination findings may influence the decision on RSI drugs, including signs of increased intracranial pressure, status epilepticus, bronchospasm, and hemodynamic instability. These findings and their implications are discussed in further detail later. Facial anomalies, burns, or trauma suggest that obtaining an adequate seal on bag-valve mask will be difficult. Children with a prominent occiput or short or inflexible neck are difficult to position optimally for BMV and laryngoscopy, and measures should be taken to optimize positioning for these patients. The airway proceduralist should anticipate difficulty with laryngoscopy in patients with small mouths or mandibles, palate abnormalities, or large tongues.50
Hoarseness, stridor, drooling, or tripod position with respiratory distress are signs of upper airway obstruction; in this scenario, the clinician should keep the child as calm as possible to prevent worsening of the obstruction and discuss the issue with the otolaryngology or anesthesia specialists for possible intubation in the operating room if available. If they are unavailable, the clinician should prepare for a possible cricothyrotomy depending on the level of airway swelling.11,49
Protection and Positioning
The ideal positioning for ventilation and intubation is alignment of the oral, pharyngeal, and tracheal axes. In infants, a rolled towel underneath the shoulders may be placed to overcome the prominent occiput causing flexion of the neck. In children, a rolled towel underneath the occiput of the head optimizes neck extension to best align the airway axes and optimize the view for the laryngoscopist.50
When there is concern for a cervical spinal cord injury, manual in-line stabilization should be maintained without extension of the head or neck during laryngoscopy, which may make visualization of the cords more difficult.29
Preoxygenation
Once the decision is made to intubate and materials have been gathered, preoxygenation with 100% oxygen should be provided as soon as possible. This technique boosts oxygen reservoirs in the body to slow progression of desaturation during the apneic period of endotracheal intubation. The goal of preoxygenation is to bring the patient’s oxygen saturation as near as possible to 100% and to denitrogenate the residual lung capacity and maximize oxygen storage of the lungs. Preoxygenation provides a longer safe apneic time period during ETT placement.51 Because of infants’ and children’s higher oxygen consumption rate and lower functional residual capacity relative to adults, oxygen desaturation occurs much more rapidly in the pediatric population. Therefore, preoxygenation becomes an exceedingly important step of RSI in infants and young children.5
For spontaneously breathing patients, apply a nonrebreather mask or place the bag valve mask over the mouth and nose without pressing into the face of the patient. Oxygen should be administered at the highest concentration available. For apneic patients, provide BMV with an oxygen source at 100% FiO2 at a rate of more than 7 L/minute. Continue to provide preoxygenation with BMV as sedatives and paralytics are administered until the patient is ready for intubation once RSI drugs have taken full effect.49 During sedation and paralysis, provide apneic oxygenation via standard nasal cannula at a flow rate of 1 L/min per year of age or high-flow nasal cannula with 100% FiO2 to extend the safe apneic time period.7
Pretreatment
Pretreatment prior to sedative and paralytic agents during RSI is optional but may be beneficial in certain clinical scenarios. Atropine, lidocaine, and fentanyl all are options that may be used to optimize conditions during the endotracheal intubation.
Atropine
A medication that often is used as a pretreatment prior to intubation in infants younger than 1 year of age is atropine. Atropine is an antimuscarinic that works through competitive inhibition of postganglionic acetylcholine receptors and direct vagolytic action, leading to parasympathetic inhibition of acetylcholine receptor in smooth muscle to allow for sympathetic stimulation to predominate. The dose for atropine during RSI is 0.02 mg/kg intravenously (IV) with a maximum dose of 1 mg.52
Infants and young children are highly susceptible to vagally induced bradycardia when undergoing laryngoscopy. Bradycardia may limit the duration of laryngoscopy and prolong the time from administering RSI medications to securing the airway with an ETT. Pretreatment with atropine limits or prevents bradycardia to allow a prolonged time for laryngoscopy and intubation. Atropine also often is used as a pretreatment when succinylcholine is planned to be used as the paralytic medication because administration of succinylcholine is associated with bradycardia and asystole in children. According to the American Heart Association guidelines, it is reasonable to administer atropine to prevent bradycardia during emergent intubations when there is a high risk for bradycardia, such as when giving succinylcholine, especially if the child requires multiple doses. Otherwise, data have shown very limited use and effectiveness of atropine in the prevention of bradycardia during intubation, and it is not routinely recommended for RSI in children.53 Administering atropine as a pretreatment limits the physician’s ability to assess oxygenation, heart rate, and neurologic status after intubation because of the prolonged effects on the sympathetic nervous system lasting approximately one hour.52
Lidocaine
Lidocaine given in an intravenous form is used as part of the RSI pretreatment protocol in many institutions, although limited evidence exists regarding its usefulness in clinical settings. Administration of lidocaine depresses the hypertensive response during laryngoscopy and has been shown to decrease intracranial pressure in patients with traumatic brain injury as well as seizing in patients with status epilepticus.54 Additionally, lidocaine has been shown to relax airways in patients with status asthmaticus.55 Complications of lidocaine administrations are rare but include arrhythmias, methemoglobinemia, and cardiovascular instability. The dose for lidocaine is 1 mg/kg to 2 mg/kg, with a maximum dose of 100 mg.56
Fentanyl
Fentanyl also may be used as pretreatment when there is concern for increased intracranial pressure by helping to blunt the sympathetic response to intubation. Similar to lidocaine, limited evidence exists regarding its usefulness in clinical practice.55 Caution should be taken when using this medication because it may cause hemodynamic collapse in a patient who is already hypotensive.57 The dose for fentanyl pretreatment is 3 mcg/kg.
Hemodynamics
In an ideal situation, hemodynamically unstable patients should be optimized prior to RSI because peri-intubation hypotension is associated with increased incidence of peri-intubation cardiac arrest. Administration of medications to help the child’s hemodynamic status, including IV fluids and push-dose vasopressors, should be considered.
IV Fluids
Many of the sedative medications administered during induction for intubation and after intubation have adverse effects of hemodynamic instability because of decreased ventricular return and myocardial depression. Additionally, the positive intrathoracic pressure provided by ventilation may further decrease venous preload. Administering a fluid bolus helps to combat these adverse effects by increasing preload and venous return.24,58 While not formally considered a pretreatment, administering a fluid bolus prior to RSI of a child may be beneficial to maintain hemodynamic stability in hemodynamically stable patients to prevent post-intubation hypotension.59
However, the effectiveness of fluid bolus administration for preventing peri-intubation cardiac arrest rarely has been studied in the pediatric population and has not been shown to reduce rates of cardiovascular collapse in critically ill adults.60 The dose for a pediatric fluid bolus is 20 mL/kg of normal saline or lactated Ringers.
Push-Dose Vasopressors
Although there is limited published evidence on the subject matter, push-dose pressors may be used as a temporizing measure prior to the initiation of a vasopressor infusion in a hypotensive patient peri-intubation. Providers should follow any hospital/department guidelines regarding the use of push-dose vasopressors. Vials of push-dose vasopressors at the appropriate dose for the child’s weight should be prepared and readily available to be administered while intubating a child, since medications given during RSI may cause hemodynamic collapse. Additionally, consider administering a dose of push-dose vasopressor prior to intubation in patients who are significantly hypotensive prior to RSI or in children with baseline poor cardiac function and who are at higher risk for hemodynamic collapse. Epinephrine is the most commonly used push-dose vasopressor in children. A push dose of epinephrine is prepared by drawing 1 mL of 1:10,000 epinephrine and diluting with 9 mL normal saline to create a 1:100,000 solution or 10 mcg/mL. The weight-based dose of this diluted solution is 0.1 mL/kg, or 1 mcg/kg.61
Paralysis and Induction
In rapid sequence intubation, sedation agents are administered first, followed closely by the paralytic agent.40 The sedation agent acts to provide amnesia, induce a state of unconsciousness, and minimize sympathetic response to intubation. The paralytic agent acts to achieve full muscle relaxation to facilitate rapid tracheal intubation. Paralytics do not provide sedation or analgesia, so sedation must be initiated prior to administration of the paralytic agent and maintained for the length of action of the paralytic. Many pharmacologic options exist for both sedative and paralytic medications, and the practitioner should choose RSI medications based on the clinical scenario. Options for sedative agents include etomidate, ketamine, propofol, and midazolam.
Etomidate
Etomidate is an ultra short-acting non-barbiturate hypnotic anesthetic agent. The dose for etomidate during RSI is 0.3 mg/kg. The time of onset is approximately 15 to 45 seconds after administration, and the duration of effect is 10 to 12 minutes.62 Because etomidate is very short-acting, the patient will require anxiolysis or analgesia shortly after intubation.
Etomidate preserves hemodynamic stability and is especially useful in patients with hypovolemic shock and hypotensive patients with status epilepticus. Etomidate also has been shown to reliably decrease intracranial pressure (ICP) and cerebral metabolic rate, making the drug a good choice for sedation in patients with increased ICP.62,63 The most widely known side effect of etomidate is transient adrenocortical suppression; for this reason, etomidate should not be used routinely in children with septic shock.64
Ketamine
Ketamine is a dissociative anesthetic derived from phencyclidine. The dose for ketamine administration in RSI is 1 mg/kg to 2 mg/kg. The time of effect is 45 to 60 seconds, with a duration of action of 10 to 20 minutes.65
Ketamine preserves protective airway reflexes and respiratory drive. Ketamine acts as a cardiovascular stimulant and is less likely to cause hemodynamic instability in patients with septic shock; therefore, it is the sedative agent of choice for patients with septic shock.66 Ketamine also acts as a bronchodilator, making it the preferred agent for intubation of status asthmaticus. A potential adverse effect of ketamine is increased ICP, so it often is avoided in patients with head injuries or concern for elevated ICP.65 Although ketamine once was believed to increase ICP and often is avoided in patients with head injuries or concern for elevated ICP, there is limited evidence to support the increase in ICP, and the provider should weigh the benefits of its use in these situations.66,67
Propofol
Propofol is a highly lipid soluble non-barbiturate sedative-hypnotic that acts on gamma-aminobutyric acid (GABA) receptors to induce sedation and amnesia. The rapid push dose of propofol for RSI is 1 mg/kg to 1.5 mg/kg. The onset of effect is 15-45 seconds, and the duration of effect of five to 10 minutes. Given that a propofol push dose is relatively short acting, the patient will require ongoing anxiolysis or analgesia shortly after intubation.
Propofol push dose is a good agent of choice in patients with status epilepticus who are hemodynamically stable because of its GABA agonist activity in the central nervous system. Adverse effects of propofol include vasodilation and myocardial depression leading to hypotension, and it should not be used for patients who are hemodynamically unstable.69
Midazolam
Midazolam is a rapidly acting benzodiazepine that binds the GABA receptor to induce amnesia and sedation. The dose of midazolam for RSI is 0.2 mg/kg to 0.3 mg/kg IV. The onset of effect is two to three minutes, with a duration of action of 30 to 45 minutes. Midazolam has anticonvulsant properties, so it may be useful in the intubation of a hemodynamically stable patient with status epilepticus. However, midazolam is rarely used as a first-line sedative agent for RSI. Adverse effects of midazolam include respiratory depression and possible induction of apnea prior to paralytic, limiting the effectiveness of preoxygenation. Midazolam also has a depressive effect on the cardiovascular system and can result in hypotension and hemodynamic instability; it should not be used in hemodynamically unstable patients.70
Sedation Choice for Specific Clinical Scenarios
For patients with hypotension other than septic shock, etomidate is the sedative agent of choice because of its preservation of hemodynamic stability. For patients with hypotension caused by septic shock, ketamine should be the first-line sedative agent, given etomidate’s potential for adrenal suppression.
For patients with increased intracranial pressure, etomidate should be the sedative of choice; propofol also is an appropriate second-line sedative agent. For hypotensive patients with a head injury, the sedative agent of choice should be etomidate. For status asthmaticus, the sedative agent of choice should be ketamine because of its bronchodilator effects; etomidate also is an appropriate choice. For hemodynamically stable patients with status epilepticus, midazolam or propofol may be used. For hypotensive patients in status epilepticus, etomidate should be given because of the potential for worsening of hemodynamic instability with propofol or midazolam.62-65,68-70
Paralytic Agents
Options for paralytic agents include rocuronium and succinylcholine.
Succinylcholine
Succinylcholine is s depolarizing paralytic agent that binds directly to the postsynaptic acetylcholine receptors of the motor endplate to cause continuous stimulation of acetylcholine receptors, resulting in transient muscular fasciculations followed by paralysis. The dose of succinylcholine is 1.5 mg/kg to 2.0 mg/kg in children weighing less than 10 kg and 1.0 mg/kg to 1.5 mg/kg in children weighing more than 10 kg. The time to effect is 30 to 60 seconds, and the duration of action is four to six minutes.
Administration of succinylcholine in infants and children has been shown to be associated with bradycardia and asystole after administration, so pretreatment with atropine is recommended in children undergoing RSI with succinylcholine, as previously discussed. The risk for bradycardia increases with each dose of succinylcholine, so repeated doses of succinylcholine should be avoided. Succinylcholine is contraindicated in multiple clinical scenarios because of adverse effects. Absolute contraindications to succinylcholine include neuromuscular disease, such as Becker or Duchenne muscular dystrophy and cerebral palsy with paralysis, severe burns, crush injuries and severe traumas, rhabdomyolysis, history of malignant hyperthermia in patient or relatives, and hyperkalemia. Relative contraindications include increased intracranial pressure, increased intraocular pressure, and known pseudocholinesterase deficiency.71
Rocuronium
Rocuronium is a nondepolarizing paralytic agent that acts as a competitive antagonist at the nicotinic cholinergic receptor. The dose of rocuronium for RSI is 1 mg/kg. The onset of action is 30-60 seconds, and the duration of action is 30 to 40 minutes.72
Rocuronium is the preferred paralytic agent of choice by many pediatric practitioners because of the many adverse effects and contraindications to succinylcholine and rocuronium’s higher safety profile.73 However, rocuronium has a much longer duration of action than succinylcholine, so succinylcholine may be considered over rocuronium in clinical scenarios where difficult intubation is anticipated.
Sugammadex is a reversal agent for rocuronium that binds rocuronium molecules and rapidly reverses the paralytic effect. This medication is incredibly useful, given the prolonged duration of action of rocuronium, although it is not readily available in many pediatric emergency departments.72
Table 3 summarizes medications and their uses throughout the RSI process.
Table 3. RSI Medications by Stage |
||
| Medication | Indications | Adverse Effects |
| Pretreatment Medications for RSI | ||
Lidocaine (1 mg/kg |
|
|
Fentanyl (3 mcg/kg) |
|
|
Atropine (0.02 mg/kg) |
|
|
| Sedation Medications for RSI | ||
Etomidate (0.3 mg/kg) |
|
|
Ketamine (1 mg/kg |
|
|
Propofol (1 mg/kg |
|
|
Midazolam (0.2 mg/kg |
|
|
| Paralytic Medications for RSI | ||
Succinylcholine (1.5 mg/kg (1.0 mg/kg |
|
|
Rocuronium (1 mg/kg) |
|
|
RSI: rapid sequence intubation; ICP: intracranial pressure |
||
Endotracheal Intubation and Confirmation
The laryngoscopist should be standing at the head of the bed with all equipment prepared and ready when the RSI drugs are administered. Once the sedation and the paralytic medications have taken effect, remove the BMV and prepare for laryngoscopy and intubation. The steps of tracheal intubation are as follows.
- Open the mouth with the right hand. Use the right hand to open the mouth using the “scissor” technique by caudally pressing on the patient’s mandibular incisors with the thumb and cranially on the patient’s maxillary incisors with the second finger.39
- Hold the laryngoscope blade with the left hand and insert the blade into the oral cavity. The laryngoscope blade should be held at the base, where the blade inserts onto the handle. Insert the blade into the right side of the patient’s mouth to the left of the right hand and carefully advance the blade into the oropharynx, swiping the blade over the tongue so that it does not obstruct the view of the cords.39
- Visualize and lift the epiglottis. If using a straight Miller blade, directly lift the epiglottis to visualize the vocal cords. If using a curved MacIntosh blade, place the blade tip in the vallecula to indirectly lift the epiglottis to visualize the vocal cords. If using video laryngoscopy, insert the hyperangulated blade midline and advance slowly, identifying airway landmarks on the video laryngoscopy screen. Advance the blade into the vallecula and lift the blade to visualize the vocal cords.11
When lifting the blade, be sure to apply upward and outward force rather than rocking backward. Rocking backward with the laryngoscopy blade does not obtain the view of the vocal cords and often results in damage to the teeth.39 - Visualize the arytenoid cartilages (vocal cords). A common error is inserting the blade too deeply; when encountering difficulty visualizing the cords, withdraw the blade slowly and allow the glottis to come into view. When intubating, a full, unobstructed view of the glottis is the optimal condition for ETT passage through the vocal cords. Suctioning may be performed to obtain a better view by removing any airway debris, secretions, or blood. Esophageal tube placement is much more likely if the glottis and vocal cords are not able to be visualized clearly.11
Given the relative cephalad positioning of the pediatric airway when compared to adults, gentle external manipulation of the larynx may help the laryngoscopist to obtain a better view of the arytenoid cartilage. The laryngoscopist should use the right hand to optimize the view and then instruct another team member to hold in the same position during intubation.48 (See Figure 5.) Applying cricoid pressure has been shown to lead to difficulty with intubation and BMV, especially in children because of their smaller airways and more flexible cartilaginous tissue, which may lead to occlusion of the trachea.9 - Advance the ETT through the arytenoid cartilages and visualize the tube and the cuff, if present, passing through the vocal cords. Remove the stylet and inflate the cuff, if present.39
The cuff should be inflated with the minimum amount of air required to prevent a cuff leak. Pediatric ETT cuff pressures should be monitored closely because high cuff pressures may result in ischemic changes to the trachea.42
The laryngoscopist should take caution not to advance the ETT too far, especially in young children with smaller airways. A useful calculation to predetermine the depth at which the ETT should be placed in children is multiplying the internal diameter of the tube by 3 for the optimal tube depth at the lips as such: Tube internal diameter (mm) × 3 = depth of insertion at the lips (cm). Another formula that may be used is: (Age in years / 2) + 12 = depth of insertion at the lips (cm).74 The Broselow tape and other length-based systems also can be used to plan the depth of ETT insertion based on the child’s height.40
Difficulty with passing the tube through the cords may be encountered if the laryngoscopist fails to maintain the optimal view, if excessive cricoid pressure is applied, if the ETT is too large, or if the shaping of the flexible stylet is suboptimal. Consider switching to a smaller ETT or altering the shape of the stylet to better suit the airway. Limit each intubation attempt to no more than 30 seconds. In between attempts, use a BMV to reoxygenate the patient and minimize desaturation. Multiple intubation attempts are associated with adverse events, including cardiac arrest.75 - Confirm ETT placement. Directly visualizing the tube pass through the vocal cords is the best method for confirming successful placement, although this form of visualization may be made difficult by blood or secretions in the airway, a small oropharynx, or a difficult laryngoscopic view. Listen for bilateral breath sounds and the absence of epigastric sounds. Confirm placement with capnography or colorimetric carbon dioxide detector. Capnography measures levels of expired carbon dioxide; clear regular waveforms on the monitor or carbon dioxide measurements of more than 30 mmHg indicate tracheal placement of the tube. Colorimetric end-tidal carbon dioxide detectors use pH paper that changes from yellow to purple with exposure to carbon dioxide to confirm tracheal tube placement.75 For children weighing less than 15 kg, a small-sized colorimetric end-tidal carbon dioxide detector should be used. For children weighing more than 15 kg, the adult-sized device should be used.76
Bedside ultrasound also may be used to confirm ETT placement. Place the probe above the cricoid membrane using a transverse view. If the tube is in the trachea, the tube will be visualized just underneath the tracheal cartilage. If the tube is in the esophagus, two separate structures will be seen side-by-side, known as the “double track sign.”44 - Secure the ETT. Once the ETT is placed and confirmed to be in the trachea, the tracheal tube should be secured at the mouth with commercially available devices or with skin adhesive tape attached securely to the maxilla. Infants have a shorter distance between glottic opening and termination of the ETT and are prone to displacement of the tip of the ETT into the oropharynx with head extension and right mainstem bronchus with neck flexion.77
Figure 5. Laryngoscopic View of the Vocal Cords |
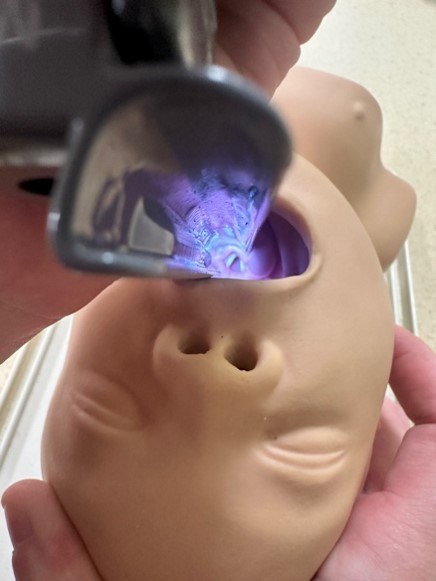 |
Courtesy of Dr. Alexandra Armato and Dr. Daniel Migliaccio. |
Post-Intubation Ventilator Management
Once the ETT has been placed and confirmed with carbon dioxide detectors, the tube should be attached to a ventilator machine, and the ventilator setting should be set based on the patient’s clinical presentation. The most commonly used ventilation setting after intubating in the emergency department is volume control. Tidal volume, PEEP, and respiratory rate may be changed as indicated by the clinical scenario. Once a ventilator mode is set, constant monitoring of ventilatory and oxygenation status should occur to guide ventilator mode adjustments.78
For children with normal lung mechanics who were intubated for altered levels of consciousness, a tidal volume of 6 mL/kg to 8 mL/kg should be used with a PEEP of 5 cm H2O and a respiratory rate that corresponds with normal parameters for the child’s age. If there is concern for intracranial hypertension, the rate should be targeted to achieve an end-tidal carbon dioxide measurement of 35 mmHg to 40 mmHg to minimize intracranial pressure.79 If there is concern for metabolic acidosis, such as in diabetic ketoacidosis, the rate should be increased to allow for respiratory compensation and to avoid worsening acidosis.78
For children with obstructive lung disease, such as asthma, a tidal volume of 6 mL/kg to 8 mL/kg should be used with a PEEP of 5 cm H2O and a slower respiratory rate of 10 to 14 breaths per minute to allow increased expiratory time. The plateau pressure should be kept at a maximum of 30 cm H2O to avoid barotrauma. Ventilation after intubation in children with asthma can be difficult because of severe bronchospasm and airway inflammation, leading to increased resistance in the circuit and increased risk of barotrauma. Breath stacking leads to dynamic hyperinflation and auto-PEEP. A slower set respiratory rate and increased expiratory time are required to prevent breath stacking.78
The lung protective strategy for ventilation is used in acute respiratory distress syndrome. This ventilator strategy improves gas exchange while reducing the risk of ventilator-induced lung injury. The tidal volume should be lower than normal at 4 mL/kg to 6 mL/kg, with a higher PEEP at 8 cm H2O to 10 cm H2O. The rate may need to be adjusted at a faster parameter than normal for the patient’s age, given the lower tidal volumes to avoid hypercapnia.78,80
Additionally, the child should be provided with adequate sedation and analgesia after intubation before the effects of the sedative and paralytic agents wear off. Sedation options include benzodiazepines such as lorazepam or midazolam and propofol. Fentanyl commonly is used in combination with a sedative to provide analgesia.78
After intubation and securing the tube, a chest X-ray should be obtained to confirm proper depth placement of the tube. The tube should be in the trachea just above the carina. Advance or retract the tube as needed.48
The head of bed should be elevated to 30 degrees unless otherwise contraindicated for cervical spine injuries. Nonviolent restraints may be required to prevent the child from pulling out the ETT or other medical equipment. An arterial blood gas should be checked 30 minutes after intubation to help guide ventilator management.78
High-Risk Populations for Intubation
Patients with certain conditions may be at risk for further deterioration during standard RSI. In some cases, modifications should be made to the intubation process in these cases to prevent exacerbation of the pathophysiologic processes associated with the conditions.
Hemodynamically Unstable Patients
Hypotensive and hemodynamically unstable patients are at risk for further decompensation during and after endotracheal intubation. In patients with hemodynamic compromise, etomidate or ketamine should be chosen as the induction agent, since these medications preserve hemodynamic stability. Because of the risk for adrenal suppression with etomidate administration in patients with septic shock, ketamine should be the induction agent of choice in patients with sepsis. Prior to intubation, the provider may give an IV fluid bolus to improve the patient’s hemodynamic status. Push-dose vasopressors should be readily available to be administered for worsening hemodynamic instability. Additionally, providers should consider a dose of push-dose vasopressor prior to induction in patients who are already significantly hypotensive while awaiting vasopressor infusions or in children with baseline poor cardiac function and at higher risk for hemodynamic collapse.81-83
After intubation, the ventilator settings should provide the lowest possible PEEP to provide oxygenation to the patient, since positive pressure ventilation decreases venous return because of the increased intrathoracic pressure and, in turn, reduces preload and cardiac output, leading to hemodynamic instability. The choice for post-intubation sedation should be an option that will optimize hemodynamics — propofol and fentanyl may aggravate hemodynamic compromise. The clinician should initiate pressure support with vasopressors, if indicated.
Increased Intracranial Pressure
Laryngoscopy and intubation can cause reflex sympathetic activation, resulting in hypertension and tachycardia caused by stimulation of the oropharynx. Increased mean arterial pressure results in increased intracranial pressure, so the provider should take measures to minimize the sympathetic effect in patients with concern for increased intracranial pressure, especially patients with suspected traumatic brain injury. In these patients, providers should consider pretreatment with IV lidocaine or fentanyl to blunt the sympathetic response. The sedation agent of choice should be targeted to avoid hypotension, which leads to decreased cerebral perfusion pressure but also avoids increasing the intracranial pressure. Etomidate is the first-line agent for patients with traumatic brain injury. As it has been demonstrated to be one of the most hemodynamically neutral sedating agents, etomidate is typically considered first line for intubation in children with traumatic head injuries.84,85
Prior to intubation, perform and document a baseline neurologic exam to include Glasgow Coma Scale scoring, pupil checks, and lateralizing sign observations. Intubation should be planned with the highest chance of first-pass success to minimize laryngeal manipulation and oropharyngeal stimulation to limit sympathetic activation, and prolonged apnea time should be avoided because hypoxia can worsen the traumatic brain injury.
After intubation, the neurological examination should be rechecked after the resolution of the neuromuscular blockade. A previous recommendation for patients with traumatic brain injury was hyperventilation. However, studies have shown that this ventilation technique should not routinely be performed for children with traumatic brain injury because hyperventilation leads to cerebral vasoconstriction with reduced cerebral blood flow and cerebral oxygenation and can lead to cerebral ischemia. The respiratory rate may be altered to achieve a target end-tidal carbon dioxide measurement of 35 mmHg to 40 mmHg to minimize intracranial pressure. The choice for post-intubation sedation should ensure hemodynamic stability and have rapid onset and offset to allow frequent neurologic examination.
Difficult Airways
A difficult airway is defined as an airway that is anticipated to be either be difficult to ventilate with BMV or difficult to intubate. In patients with anticipated difficult airways, consideration of a backup plan is extremely important. Equipment for difficult airway management should be available at the bedside.11 In certain cases, the most appropriate action may be to keep the child calm until the patient can be taken to the operating room for intubation with anesthesia or otolaryngology. The management of a difficult airway is a broad topic, which will be discussed in the next issue of Pediatric Emergency Medicine Reports.
Ventilation Troubleshooting
If decompensation occurs after successful endotracheal intubation and difficulty is encountered with ventilating or oxygenating the patient, the mnemonic DOPES (displacement, obstruction, pneumothorax, equipment, stomach) is useful to guide evaluation and treatment.49
ETT Displacement
The clinician should listen to the lungs for bilateral breath sounds and observe chest wall for chest rise. The equipment should be checked for disconnection in the circuit. Repeated chest X-rays should be obtained to confirm ETT placement at the appropriate depth at approximately 1 cm to 2 cm above the carina.
Obstruction of ETT
Air flow through the ETT can become obstructed by respiratory secretions, blood, tracheal wall, or tracheal foreign body. The clinician or respiratory therapist should attempt suctioning through the ETT to remove secretions. The ETT should be evaluated for any kinks, and, if applicable, a bite block should be placed to prevent the patient from biting down on the ETT.
Pneumothorax
A repeat chest X-ray should be obtained to evaluate for pneumothorax. Bedside ultrasound also may be helpful to evaluate for pneumothorax through the absence of lung sliding or identification of a lung point. If a pneumothorax is identified, the patient should undergo urgent tube thoracostomy.
Equipment Problems
If equipment failure is suspected, the patient should be disconnected from the ventilator and manually ventilated with 100% FiO2 until the ventilator is replaced.
Stomach
Increased abdominal pressure caused by insufflation during BMV use can impair ventilation of the lungs through the ETT. A nasogastric or orogastric tube should be placed to allow for release of air within the stomach.
Conclusion
Pediatric airways can be very challenging given the anatomy and size of children. However, being equipped with understanding of pediatric airway basics will prepare the provider to handle pediatric airway emergencies with confidence and skill. The next issue of Pediatric Emergency Medicine Reports will review more advanced airway management scenarios.
Alexandra Armato, MD, is is a resident at the Department of Emergency Medicine at University of North Carolina at Chapel Hill.
Daniel Migliaccio, MD, FPD, FAAEM, is a clinical associate professor, division director of emergency ultrasound, ultrasound fellowship director, Department of Emergency Medicine, University of North Carolina at Chapel Hill.
References
- Harless J, Ramaiah R, Bhananker SM. Pediatric airway management. Int J Crit Illn Inj Sci. 2014;4(1):65-70.
- Adewale L. Anatomy and assessment of the pediatric airway. Pediatr Anesth. 2009;19(Suppl 1):1-8.
- Alfahel W, Gopinath A, Arheart KL, et al. The effects of a shoulder roll during laryngoscopy in infants: A randomized, single-blinded, crossover study. Anesth Analg. 2020;131(4):1210-1216.
- Mace SE, Khan N. Needle cricothyrotomy. Emerg Med Clin North Am. 2008;26(4):1085-1101, xi.
- Saikia D, Mahanta B. Cardiovascular and respiratory physiology in children. Indian J Anaesth. 2019;63(9):690-697.
- Fuchs A, Koepp G, Huber M, et al. Apnoeic oxygenation during paediatric tracheal intubation: A systematic review and meta-analysis. Br J Anaesth. 2024;132(2):392-406.
- Napolotino N, Polikoff L, Edwards L, et al. Effect of apneic oxygenation with intubation to reduce severe desaturation and adverse tracheal intubation-associated events in critically ill children. Crit Care. 2023;27(1):26.
- Tranchsel D, Erb TO, Hammer J, von Ungern-Sternberg BS. Developmental respiratory physiology. Pediatr Anesth. 2021;32(2):108-117.
- Bucher JT, Vashisht R, Ladd M, Cooper JS. Bag-valve-mask ventilation. StatPearls [Internet]. Updated May 21, 2023. https://www.ncbi.nlm.nih.gov/books/NBK441924/
- Lim SY, Pettit RS. Pharmacokinetic considerations in pediatric pharmacotherapy. Am J Health Syst Pharm. 2019;76(19):1472-1480.
- Avva U, Lata JM, Kiel J. Airway management. StatPearls [Internet]. Updated May 19, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470403/
- Bhalala US, Hemani M, Shah M, et al. Defining optimal head-tilt position of resuscitation in neonates and young infants using magnetic resonance imaging data. PLoS One. 2016;11(3):e0151789.
- Roth B, Magnusson J, Johansson I, et al. Jaw lift — a simple and effective method to open the airway in children. Resuscitation. 1998;39(3):171-174.
- Bhutta BS, Alghoula F, Berim I. Hypoxia. StatPearls [Internet]. Updated March 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482316/
- Gupta V, Cheifetz IM. Heliox administration in the pediatric intensive care unit: An evidence-based review. Pediatr Crit Care Med. 2005;6:204-211.
- Nascimiento MS, Santos É, do Prado C. Helium-oxygen mixture: Clinical applicability in an intensive care unit. Einstein (Sao Paulo). 2018;16(4):eAO4199.
- Manthous CA, Hall JB, Caputo MA, et al. Heliox improves pulsus paradoxus and peak expiratory flow in non-intubated patients with severe asthma. Am J Respir Crit Care Med. 1995;151(2 Pt 1):310-314.
- Liet, J-M, Ducruet T, Gupta V, Cambione G. Heliox inhalation therapy for bronchiolitis in infants. Cochrane Database Syst Rev. 2015;2015(9):CD006915.
- Kwon J-W. High-flow nasal cannula oxygen therapy in children: A clinical review. Clin Exp Pediatr. 2019;63(1):3-7.
- Hough JL, Pham TMT, Schibler A. Physiologic effect of high-flow nasal cannula in infants with bronchiolitis. Pediatr Crit Care Med. 2014;15(5):e214-e219.
- Sharma S, Danckers M, Sanghavi DK, Chakraborty RK. High-flow nasal cannula. StatPearls [Internet]. Updated April 6, 2023. https://www.ncbi.nlm.nih.gov/books/NBK526071/
- Haut C. Pediatric noninvasive ventilation. J Pediatr Intensive Care. 2015;4(2):121-127.
- Thia LP, McKenzie SA, Blyth TP, et al. Randomised controlled trial of nasal continuous positive airways pressure (CPAP) in bronchiolitis. Arch Dis Child. 2007;93(1):45-47.
- Potchileev I, Doroshenko M, Mohammed AN. Positive pressure ventilation. Updated Jan. 30, 2023. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK560916/
- Williams AM, Abramo TJ, Shah MV, et al. Safety and clinical findings of BiPAP utilization in children 20 kg or less for asthma exacerbations. Intensive Care Med. 2011;37(8):1338-1343.
- Dafilou B, Schwester D, Ruhl N, Marques-Baptista A. It’s in the bag: Tidal volumes in adult and pediatric bag valve masks. West J Emerg Med. 2020;21(3):722-726.
- O’Donnell CPF, Davis PG, Lau R, et al. Neonatal resuscitation 3: Manometer use in a model of face mask ventilation. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F397-F400.
- Driver BE, Atkins AH, Reardon RF. The danger of using pop-off valves for pediatric emergency airway management. J Emerg Med. 2020;59(4):590-592.
- Austin N, Krishnamoorthy V, Dagal A. Airway management in cervical spine injury. Int J Crit Illn Inj Sci. 2014;4(1):50-56.
- Hart D, Reardon R, Ward C, Miner J. Face mask ventilation: A comparison of three techniques. J Emer Med. 2013;44(5):1028-1033.
- Davidovic L, LaCovey D, Pitetti RD. Comparison of 1- versus 2-person bag-valve-mask techniques for manikin ventilation of infants and children. Ann Emerg Med. 2005;46(1):37-42.
- Ujevich MM, Pozun A. Pediatric and neonatal resuscitation. StatPearls [Internet]. Updated March 8, 2023. https://www.ncbi.nlm.nih.gov/books/NBK572069/
- Atanelov Z, Aina T, Smith T, Rebstock SE. Nasopharyngeal airway. StatPearls [Internet]. Updated Jan. 30, 2024. https://www.ncbi.nlm.nih.gov/books/NBK513220/
- Johnson M, Miscovic A, Ray A, et al. The nasopharyngeal airway: Estimation of the
nares-to-mandible and nares-to-tragus distance in young children to assess current clinical practice. Resuscitation. 2019;140:50-54. - Castro D, Freeman LA. Oropharyngeal airway. StatPearls [Internet]. Updated July 31, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470198/
- Tume LN, Copnell B. Endotracheal suctioning of the critically ill child. J Pediatr Intensive Care. 2015;4(2):56-63.
- Garcia-Marcinkiewicz AG, Lee LK, Haydar B, et al. Difficult or impossible facemask ventilation in children with difficult tracheal intubation: A retrospective analysis of the PeDI registry. Br J Anaesth. 2023;131(1):178-187.
- Sagarin MJ, Chang V, Sakles JC, et al. Rapid sequence intubation for pediatric emergency airway management. Pediatr Emerg Care. 2002;18(6):417-423.
- Schrader M, Urits I. Tracheal rapid sequence intubation. StatPearls [Internet]. Updated Oct. 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK560592/
- Borkar N, Sharma C, Francis J, et al. Applicability of the Broselow pediatric emergency tape to predict the size of endotracheal tube and laryngeal mask airway in pediatric patients undergoing surgery: A retrospective analysis. Cureus. 2023;15(1):e33327.
- Miller KA, Dechnik A, Miller AF, et al. Video-assisted laryngoscopy for pediatric tracheal intubation in the emergency department: A multicenter study of clinical outcomes. Ann Emerg Med. 2022;81(2):113-122.
- Ahmed RA, Boyer TJ. Endotracheal tube. StatPearls [Internet]. Updated July 24, 2023. https://www.ncbi.nlm.nih.gov/books/NBK539747/
- Park S, Shin SW, Kim HJ, et al. Choice of the correct size of endotracheal tube in pediatric patients. Anesth Pain Med (Seoul). 2022;17(4):352-360.
- Liu Y, Ma W, Liu J. Applications of airway ultrasound for endotracheal intubation in pediatric patients: A systematic review. J Clin Med. 2023;12(4):1477.
- Passi Y, Sathyamoorthy M, Lerman J, et al. Comparison of the laryngoscopy views with the size 1 Miller and MacIntosh laryngoscope blades lifting the epiglottis or the base of the tongue in infants and children < 2 yr of age. Br J Anaesth. 2014;113(5):869-874.
- Mellick LB, Edholm T, Corbett SW. Pediatric laryngoscope blade size selection using facial landmarks. Pediatr Emerg Care. 2006;22(4):226-229.
- Swaika S, Ghosh S, Bhattacharyya C. Airway devices in paediatric anaesthesia. Indian J Anaesth. 2019;63(9):721-728.
- Alvarado AC, Panakos P. Endotracheal tube intubation techniques. Updated July 10, 2023. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK560730/
- Karisik M, Vulovic T, Simic D. Pediatric airway management: Steps through the time. J Surg. 2022;7:1472.
- Vialet R, Nau A, Chaumoître K, Martin C. Effects of head posture on the oral, pharyngeal and laryngeal axis alignment in infants and young children by magnetic resonance imaging. Paediatr Anaesth. 2008;18(6):525-531.
- Danish MA. Pre-oxygenation and anesthesia: A detailed review. Cureus. 2021;13:e13240.
- McLendon K, Preuss CV. Atropine. StatPearls [Internet]. Updated June 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470551/
- De Caen AR, Berg MD, Chameides L, et al. Part 12: Pediatric advanced life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S526-S542.
- Adamzik M, Groeben H, Farahani R, et al. Intravenous lidocaine after tracheal intubation mitigates bronchoconstriction in patients with asthma. Anesth Analg. 2007;104(1):168-172.
- Kuzak N, Harrison DW, Zed PJ. Use of lidocaine and fentanyl premedication for neuroprotective rapid sequence intubation in the emergency department. CJEM. 2006;8(2):80-84.
- Beecham GB, Nessel TA, Goyal A. Lidocaine. StatPearls [Internet]. Updated Aug. 16, 2024. https://www.ncbi.nlm.nih.gov/books/NBK539881/
- Takahashi J, Goto T, Okamoto H, et al. Association of fentanyl use in rapid sequence intubation with post-intubation hypotension. Am J Emerg Med. 2018;36(11):2044-2049.
- Jaber S, Jung B, Corne P, et al. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: A prospective, multiple-center study. Intensive Care Med. 2010;36(2):248–255.
- Lu Z, Guo J, Zhang A, et al. Fluid infusion prior to intubation or anesthesia: A meat-analysis of randomized controlled trials. J Crit Care. 2024;84:154881.
- Heckle T, Kerrey BT, Zhang Y, Dean P. Interventions to reduce risk in the physiologically difficult pediatric airway: A descriptive study and exploratory analysis. J Am Coll Emerg Physicians Open. 2025;6(2):100052.
- Ross CE, Asaro LA, Wypij D, et al. Physiologic response to pre-arrest bolus dilute epinephrine in the pediatric intensive care unit. Resuscitation. 2018;126:137-142.
- Williams LM, Boyd KL, Fitzgerald BM. Etomidate. StatPearls [Internet]. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK535364/
- Guldner G, Schultz J, Sexton P, et al. Etomidate for rapid-sequence intubation in young children: Hemodynamic effects and adverse events. Acad Emerg Med. 2003;10(2):134-139.
- den Brinker M, Joosten KF, Liem O, et al. Adrenal insufficiency in meningococcal sepsis: Bioavailable cortisol levels and impact of interleukin-6 levels and intubation with etomidate on adrenal function and mortality. J Clin Endocrinol Metab. 2005;90(9):5110-5117.
- Rosenbaum SB, Gupta V, Patel P, et al. Ketamine. StatPearls [Internet]. Updated Jan. 30, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470357/
- Kochanek PM, Herrmann JR, Bleck TP. The evolution of ketamine in severe pediatric traumatic brain injury, from contraband to promising neuroprotectant? Crit Care Med. 2023;51(5):677.
- Laws JC, Vance EH, Betters KA, et al. Acute effects of ketamine on intracranial pressure in children with severe traumatic brain injury. Crit Care Med. 2023;51(5):563.
- Srivilaithon W, Bumrungphanithaworn A, Daorattanachai K, et al. Clinical outcomes after a single induction dose of etomidate versus ketamine for emergency department sepsis intubation: A randomized controlled trial. Sci Rep. 2023;13(1):6362.
- Folino TB, Muco E, Safadi AO, Parks LJ. Propofol. StatPearls [Internet]. Updated July 24, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430884/
- Lingamchetty TN, Hosseini SA, Saadabadi A. Midazolam. StatPearls [Internet]. Updated June 5, 2023. https://www.ncbi.nlm.nih.gov/books/NBK537321/
- Hager HH, Burns B. Succinylcholine chloride. StatPearls [Internet]. Updated Feb. 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK499984/
- Jain A, Wermuth HR, Dua A, et al. Rocuronium. StatPearls [Internet]. Updated Feb. 28, 2024. https://www.ncbi.nlm.nih.gov/books/NBK539888/
- Kumar A, Kumar A, Bharti AK, et al. A randomized double-blind comparative study of the intubating conditions and hemodynamic effects of rocuronium and succinylcholine in pediatric patients. Cureus. 2023;15(9):e44631.
- American Heart Association. Part 10: Pediatric advanced life support. Circulation. 2000;102(1):291-342.
- Yang T, Shao S, Lee Y, et al. Risk factors for peri-intubation cardiac arrest: A systematic review and meta-analysis. Biomed J. 2024;47(3):100656.
- Garey DM, Ward R, Rich W, et al. Tidal volume threshold for colorimetric carbon dioxide detectors available for use in neonates. Pediatrics. 2008;121(6):e1524-e1527.
- Weiss M, Knirsch W, Kretschmar O, et al. Tracheal tube-tip displacement in children during head-neck movement — a radiologic assessment. Br J Anaesth. 2006;96(4):486-491.
- Mora Carpio AL, Mora JI. Ventilator management. StatPearls [Internet]. Updated March 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK448186/
- Asehnoune K, Roquilly A, Cinotti R. Respiratory management in patients with severe brain injury. Crit Care. 2018;22(1):76.
- Wong JJM, Lee SW, Tan HL, et al. Lung-protective mechanical ventilation strategies in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med. 2020;21(8):720-728.
- Marino BS, Tabbutt S, MacLaren G, et al. Cardiopulmonary Resuscitation in Infants and Children With Cardiac Disease. A Scientific Statement from the American Heart Association.Circulation. 2018;137(22) :e691-e782.
- Angurana SK. Low-dose epinephrine boluses for acute hypotension in the PICU: Are they needed? Pediatr Crit Care Med. 2018;19(9):912.
- Holden D, Ramich J, Timm E, et al. Safety considerations and guideline-based safe use recommendations for “bolus-dose” vasopressors in the emergency department. Ann Emerg Med. 2018;71(1):83-92.
- Zuckerbraun NS, Pitetti RD, Herr SM, et al. Use of etomidate as an induction agent for rapid sequence intubation in a pediatric emergency department. Acad Emerg Med. 2006;13(6):602-609.
- Guldner G, Schultz J, Sexton P, et al. Etomidate for rapid-sequence intubation in young children: Hemodynamic effects and adverse events. Acad Emerg Med. 2003;10(2):134.