
Pediatric Abdominal Trauma
July 1, 2025
By Jordan Hickey, MD, and Charles Jang, MD
Abdominal trauma is a common occurrence, and acute care clinicians must be familiar with the evaluation and management of children with potential abdominal injuries. The authors provide a comprehensive diagnostic and therapeutic approach to this population with the goal of optimizing their outcome.
—Ann M. Dietrich, MD, Editor
EXECUTIVE SUMMARY
- Remember that because of comparatively larger untethered organs combined with a relatively high costal margin, polytrauma is more common in pediatric patients than in adults and should be considered the rule rather than the exception.
- Relying on blood pressure alone can be falsely reassuring; children may compensate with tachycardia until loss of 30% circulatory volume. Subtle secondary signs include weak peripheral pulses, skin mottling, cool extremities, and decreased response to painful stimuli. As a rough guide, the lower limit of normal for systolic blood pressure can be estimated as 90 + (age × 2) in mmHg, while hypotension can be estimated as 70 + (age × 2).
- The Shock Index Pediatric Adjusted (SIPA) score was developed for pediatrics and is considered positive if above 1.22 for patients 4-6 years of age, 1.0 for those 7-12-years of age, and 0.9 for patients 13-16 years of age. The original study showed correlation of a positive score with worse outcomes in terms of blood transfusion and in-hospital mortality. However, later studies have shown only modest sensitivity and specificity, with correlation worsening in younger age groups. SIPA score alone should not guide resuscitation but should be used in conjunction with other findings.
- A negative extended focused assessment with sonography in trauma (eFAST) exam does not exclude intra-abdominal injury. A positive exam could guide early operative management, especially in the unstable patient.
- A handlebar-type injury is classically associated with duodenal hematomas and pancreatic injury.
- If there is concern for accompanying thoracic trauma, a prompt chest X-ray should be obtained because many pediatric chest injuries requiring intervention may be diagnosed by them. If a patient is hemodynamically stable without pelvic pain or tenderness on exam, the use of routine pelvic X-ray can be deferred.
- A Pediatric Emergency Care Applied Research Network (PECARN)-derived prediction rule was developed to identify children who would be at very low risk for clinically significant intra-abdominal injury, which was defined as an injury requiring acute intervention. The following variables were included: evidence of abdominal wall trauma (including seatbelt sign), Glasgow Coma Scale score < 14, abdominal tenderness on exam, thoracic wall trauma, complaints of abdominal pain, decreased breath sounds, and vomiting. Using this rule, the patients without any of these variables present were at very low risk (0.1%) of intra-abdominal injury requiring intervention. Their sensitivity and negative predictive value were 97% and 99.9%, respectively.
- A small percentage of patients initially undergoing nonoperative management will ultimately “fail,” or require operative care during their admission, about 0.8% of the time. Factors suggesting failure of nonoperative management included older age, positive FAST, contrast extravasation on computed tomography, severe liver injury, concomitant pancreas injury, concomitant gastrointestinal injury, concomitant mesenteric injury, and higher Injury Severity Score.
Introduction
In the United States, trauma is the most common cause of death in the pediatric population.1 According to a recent single-center study, roughly 8% to 10% of pediatric trauma admissions involve injuries to the abdomen.2 The emergency department (ED) provider, no matter the setting, must be prepared to evaluate and treat traumatic injuries in those of all ages. This review seeks to provide essential steps to evaluating, treating, and providing appropriate disposition for the injured child with abdominal trauma.
Children have important anatomic and physiologic differences from adults, leading to different injury patterns and resuscitation endpoints. However, this should not get in the way of effective step-wise resuscitation or following algorithms such as Advanced Trauma Life Support (ATLS). After initial history and examination, the emergency provider (EP) should apply validated stratification tools to weigh risks and benefits of obtaining advanced imaging. If clinically significant intra-abdominal injury (IAI) is discovered, prompt consultation with a surgeon is recommended. Patients may require transfer to a pediatric trauma center even if nonoperative management is recommended.
Pathophysiology and Epidemiology
Children are predisposed to different injury patterns than those of adults. They have proportionally larger solid organs grouped closer together, leading to increased probability of multiple injuries.3,4 In addition, they have less protective adipose tissue/muscular tissue in the abdominal wall, combined with more flexible ribs and a higher costal margin. This can lead to clinically significant injury with a lower mechanism of trauma than in adults.4,5 Physiologically, they have a faster heart rate at baseline. Blood pressure can be falsely reassuring in early shock, since they are able to compensate with tachycardia until blood loss reaches 30% of their total circulating volume.3 Furthermore, a larger surface area to mass ratio leads to faster onset of hypothermia.3
Blunt injury remains the predominant mode of ED pediatric abdominal trauma cases in the United States (90%, compared to 10% penetrating).6 Common mechanisms overall according to the Centers for Disease Control and Prevention Web-based Injury Statistics Query and Reporting System (CDC WISQARS) database include falls, striking injury, cutting/piercing injury, and motor vehicle collisions (MVCs), while a single-hospital study in Florida found the mechanism among patients requiring admission included falls (45%), traffic accidents (20%), sports (8%), and assaults (4%); firearms made up 2% of their sample.2,7 Of fatal mechanisms, WISQARS reports that firearms and MVCs made up a majority of pediatric traumatic deaths in 2022.8
A retrospective cross-sectional study of 6,661 pediatric patients with IAI on computed tomography (CT) scans between 2010-2019 in 33 U.S. children’s hospitals showed that the liver (32%) and spleen (25.9%) are most commonly injured, followed by the kidney (15.2%), bowel (8.0%), pancreas (3.7%), and genitourinary organs (3.2%).9 A study evaluating penetrating torso trauma in the Trauma Quality Improvement Program (TQIP) database found 1,220 isolated abdominal gunshot wounds (GSWs) and 678 stab wounds. In contrast to blunt trauma, GSWs tend to affect the small bowel and large bowel more than the solid organs. Stab wounds most commonly affect the liver and small intestine.10
Pediatric trauma outside the United States in developed nations seems to show similar epidemiology, with a small retrospective study in Japan finding the most common mechanisms being MVCs, falls, and bike accidents, and a study of Portuguese trauma patients finding a predominance of MVCs, pedestrian injuries, and falls.11,12 Studies from Uganda, Ethiopia, and India suggest increased rates of falls and motor vehicle-vs-pedestrian accidents; a study from Saudi Arabia suggests a higher mortality rate as well compared to developed nations, which the authors suggest may be due to a lack of adherence to safety measures, such as car seat and helmet use.13-16
In previous literature, obesity had been studied as a potential protective factor in trauma. However, Alattar et al performed a systematic review of MVC blunt trauma, finding two studies without a significant difference between the rates of severe injury, while another found increased rates in obese patients in those 10 to 13 years of age.17 Additionally, Mulvihill et al performed a retrospective analysis evaluating abdominal injury in pediatric motor vehicle vs. pedestrian cases, showing an increased risk of severe injury, hospital admission, intensive care unit (ICU) admission, and increased ICU length of stay in obese pediatric patients.18 Obesity may decrease the rate of operative intervention in penetrating trauma, although whether this is related to any “armor” effect is unclear. Patients with obesity are at higher risk of increased ICU length of stay, although there does not appear to be a significant mortality difference.10,19
Initial Approach to the Critical Trauma Patient
Prehospital Care and Assessment
The initial decision to transport patients to a pediatric trauma center should be dictated by the suspected need for trauma intervention. During the initial prehospital assessment of a trauma patient, care must be taken to obtain a full set of vital signs and age-adjusted Glasgow Coma Scale (GCS) score to determine the ideal transport destination, especially because younger pediatric patients are found to have lower rates of documented vital signs.20,21 The National Expert Panel on Field Triage set out recommendations in 2021 with the goal to reduce undertriage (i.e., care at a non-trauma center facility), stating targets set at ≤ 5% for undertriage and ≤ 35% for overtriage, citing a current undertriage rate of 51% in children.22 The National Expert Panel on Field Triage lists several criteria for injuries for which transfer to the highest-level regional trauma center should be considered.
Relevant to abdominal injury, “red” or high-risk injury patterns are ones for which trauma center transport are recommended, and they include:
- All penetrating torso injuries;
- Chest wall instability/deformity or flail chest;
- Suspected pelvic fractures;
- Actively bleeding wounds requiring packing with continuous pressure.
Vital sign abnormalities include:
- Respiratory distress including bradypnea (< 10 breaths/minute) or tachypnea (> 29 breaths/minute);
- Hypoxia < 90% SpO2 on room air;
- Systolic blood pressure (SBP) < 90 mmHg or heart rate (HR) > SBP (> 10 years of age);
- SBP < 70 + age × 2 mmHg (0 to 9 years of age);
- Inability to follow commands (or motor GCS < 6).22
“Yellow” or moderate-risk patterns should preferentially lead to transfer to a trauma center. Specific pediatric considerations thereof include:
- Any child unrestrained or in an unsecured child safety seat in an MVC;
- Any low-level fall in children < 5 years of age;
- Any suspicion of child abuse.
Concerning mechanisms in all ages include:
- Auto accidents with ejection;
- Significant intrusion (> 12 inches at occupant site, > 18 inches at any site);
- Death in the same passenger compartment;
- Separation from a transport vehicle (bicycle, ATV, horse, etc.) with significant impact;
- Fall > 10 feet.22
Primary Survey
Prior to arrival, providers should obtain a length-based resuscitation tape for use to rapidly identify the correct airway equipment, volumes for resuscitation, and weight-based medication doses.3 Pediatric trauma resuscitation centers around the ABCDEs of trauma resuscitation: airway, breathing, and circulation, followed by Disability and full Exposure. An extended focused assessment with sonography in trauma (eFAST) exam should follow the primary survey to aid in identifying any significant injury.
Management of airway and breathing is beyond the scope of this review, except to caution the EP that because of comparatively larger untethered organs combined with a relatively high costal margin, polytrauma is more common in pediatric patients than in adults and should be considered the rule rather than the exception.3 Thoracic injuries should increase clinical suspicion for significant IAI.
When assessing circulation, it is important to remember that normal pediatric vital signs vary with age and a reference point for these should be readily available. As previously mentioned, relying on blood pressure alone can be falsely reassuring; children may compensate with tachycardia until loss of 30% circulatory volume.3 Subtle secondary signs include weakening peripheral pulses, narrowed pulse pressure < 20 mmHg difference between systolic and diastolic blood pressure, skin mottling, cool extremities, and decreased response to painful stimuli.3 As a rough guide, the lower limit of normal for systolic blood pressure can be estimated as 90 + (age × 2) in mmHg, while hypotension can be estimated as 70 + (age × 2).3
As a cautionary note, in adults, the shock index (heart rate:systolic blood pressure ratio) has been used as a screen for occult or compensated shock, with a value > 0.9 considered positive. The Shock Index Pediatric Adjusted score (SIPA) was developed for pediatrics and is considered positive if above 1.22 for patients 4-6 years of age, 1.0 for those 7-12-years of age, and 0.9 for patients 13-16 years of age. The original study showed correlation of a positive score with worse outcomes in terms of blood transfusion and in-hospital mortality. However, later studies have shown only modest sensitivity and specificity, with correlation worsening in younger age groups.23-25 SIPA alone should not guide resuscitation but should be used in conjunction with other findings.
When assessing for disability, the evaluating provider should be diligent in using the age-adjusted GCS in children younger than 2 years of age, since a score < 14 makes the abdominal exam unreliable and is associated with higher a risk of significant IAI.26
Following full exposure and the secondary survey, the use of heat lamps, heaters, or blankets should be used to avoid hypothermia.3
The eFAST exam is a low-sensitivity, high-specificity test that can be used to rapidly rule in intraperitoneal free fluid by examining the right upper quadrant, left upper quadrant, and suprapubic region (and additionally examines the pericardium for effusion and bilateral lungs for pneumothoraces). However, a negative eFAST exam does not exclude IAI. A positive exam could guide early operative management, especially in the unstable patient.27 Limitations of the FAST exam include inability to assess retroperitoneal or visceral injuries and operator-dependence.28
Secondary Survey
The secondary survey is a head-to-toe examination of the patient for signs of injury. Of course, abdominal pain and tenderness should raise concern for IAI, but this is unreliable in patients with GCS ≤ 13. Other signs of abdominal injury, such as bruising/ecchymosis, erythema, or abrasions, may be seen.4 Signs of peritonitis also may be appreciated on abdominal examination, such as rebound tenderness, involuntary guarding, and abdominal rigidity. Because children’s organs are proportionally larger, any associated blunt or penetrating injury to the lower chest, back, flank, pelvis, or perineum also should prompt evaluation for IAI.3,4,26 If the mechanism involves low-mechanism penetrating trauma, the consulting surgeon may perform a local wound exploration at the bedside to see if it penetrates the peritoneum. Blood at the rectum and genitals may be seen during exam, with blood at the urethral meatus or lacerations of the vaginal canal (especially if associated with pelvic fractures) suggestive of urinary tract or bladder injury.29,30 Digital rectal exam other than examination for gross blood should be reserved for consideration of rectal tone in suspected spinal injury.
Interventions in the Trauma Bay
In ATLS, life threats should be addressed as soon as they are identified. After evaluating and treating airway and breathing concerns, circulation should be addressed, meaning that vascular access is critical. If intravenous (IV) access cannot be obtained within two attempts, intraosseous access should be attempted in the proximal tibia or distal femur.3
It is a truism that hypovolemic (i.e., hemorrhagic) shock should be treated with volume, but there are key points to remember in the management of pediatric patients. As a departure from prior recommendations, early transfusion is recommended over large volumes of crystalloid.31,32 The 2018 ATLS recommendations shifted from previous editions. No longer are two 20 mL/kg crystalloid boluses administered prior to consideration of blood products. Only one 20 mL/kg bolus should be given prior to consideration of blood products. A multicenter prospective study found that in a population of pediatric trauma patients with elevated age-adjusted shock index, early transfusion led to decreased total fluid administration volume, and that increased crystalloid administration led to extended ventilator, ICU, and hospital days.33 Balanced resuscitation is recommended if large-volume blood products are required.32,34 Whole blood is recommended if available.32 As a note, permissive hypotension may benefit adult trauma patients, but the Pediatric Traumatic Hemorrhagic Shock Consensus Conference (PTHSCC) recommends against it in pediatrics, given the lack of evidence of benefit as well as the prevalence of head injuries that would be actively harmed by hypotension.31
Pelvic binders are indicated in the unstable patient with a suspected pelvic ring fracture.30 If there is concern for urethral injury with need for bladder drainage, a suprapubic catheter should be considered (if unstable) or retrograde urethrogram should be completed for further evaluation.35
Viscoelastic monitoring (VEM) in the form of thromboelastography (TEG) or rotational thrombelastometry (ROTEM) should be obtained if available to help guide resuscitation in patients with hemorrhagic shock.31
Tranexamic acid (TXA) has been shown to improve outcomes in adult traumas, but it has not been well-studied in pediatrics. Combat-based observational studies suggest a mortality benefit. The PTHSCC suggests administration within three hours with a low certainty of evidence.31
Above all, a surgeon should be consulted early if there is high suspicion for significant IAI.3 A child with hemodynamic instability despite resuscitation with blood products should go emergently to the operating room (OR).3 Signs of peritonitis in penetrating injury should go to the OR.36 If the patient is hemodynamically stable, CT imaging should be considered. In any case, per ATLS recommendations, “Nonoperative management of confirmed solid organ injuries is a surgical decision made by surgeons, just as is the decision to operate. Therefore, the surgeon must supervise the treatment of pediatric trauma patients.”3 (Emphasis added.)
History and Physical Exam
After the initial ABCDE of resuscitation, consider the “F” for “family,” since it can be beneficial for family members to witness resuscitations. The presence of a staff member who will be dedicated to the family members and who is knowledgeable about trauma resuscitative protocols can be helpful. Furthermore, family members and first responders can provide information about the mechanism, initial presentation, and the appearance of the scene.4
In evaluating the stable patient who appears fearful of examination by a medical professional, the parent can be instructed to palpate the child’s abdomen to initially assess for tenderness and guarding, prior to the provider’s examination.37 Starting with auscultation and gently pressing the stethoscope in the abdomen can assess for tenderness. While the rates of abdominal tenderness are similar between adults and children following trauma, the abdominal exam becomes less reliable in children with an initial GCS ≤ 13. Additionally, a crying child will swallow air, leading to an artificially distended abdomen.4 Skin findings, such as bruising/ecchymoses, abrasions, or erythema, increase the risk of significant IAI.4
In the following subsections, different mechanisms will be addressed with important prognostic factors to evaluate.
Motor Vehicle Collisions
Accident features that portend worse injuries include lateral force, being struck by vehicle structures such as door protuberances, seatbelt sign (bruising over the lower abdomen or across the chest where the seatbelt pressed against it), or improper car restraint for age.5
A systematic review by Giovannini et al notes that a 21% reduction in mortality risk is seen in children aged 2 to 6 years when a child restraint system is used vs. a seat belt alone. The immature pelvis cannot properly anchor the lap belt and the child’s body slides forward, allowing the belt to ride up into the belly, transmitting a large amount of force into the abdomen.5 In addition to solid organ injuries, such “seat belt injuries” can lead to hollow organ injuries (most commonly at the ligament of Treitz), along with traumatic abdominal wall hernia or even aortic injury.5
Auto-vs-Pedestrian Accidents
As previously stated, this mechanism has a higher incidence in developing nations. A retrospective study by Halari et al of fatal auto-vs-pedestrian accidents in Ontario, Canada, between 2013 and 2016 found 25 cases, of which a majority had high hood edges compared to cars, along with low-speed mechanisms involving turning at intersections. Of note, the Waddell triad of knee, femur/pelvis, and craniocerebral injuries is classically associated with frontal auto-vs-pedestrian accidents in adults, but these injuries were not commonly seen in this study.38 Injuries are mechanically based on the height at which the victim is struck, leading to adults being commonly hit in the lower extremities, sometimes followed by striking the vehicle with the head and/or torso.39 For children, however, Halari et al found that polytrauma is the rule and not the exception in motor vehicle-vs-pedestrian fatalities, describing a fatal “dyad” of cranial and thoracic injury prevalent among patients < 5 years of age; “triad” of cranial, cervical, and thoracic injury among patients 5 to 10 years of age; and “tetrad” of cranial, cervical, thoracic, and abdominal injuries in patients older than 11 years of age.38 Therefore, abdominal injury was commonly found but not isolated, showing up in 20 (80%) of their 25 cases, of which five had serious solid organ injuries identified.
Bicycle Injuries
It is imperative to have a high index of suspicion for significant IAI with patients presenting after bicycle accidents with a handlebar-type injury.40 This mechanism is classically associated with duodenal hematomas and pancreatic injury. Cheung et al found a much higher incidence of pancreaticoduodenal injury than among the general population of pediatric abdominal traumas (18.7% pancreatic, 11.9% small bowel). However, liver injury still was the most common at 25.5%.40 These patients can have delayed presentation with concerning signs for small bowel obstruction (e.g., bilious emesis) due to expanding hematoma or pancreatic/biliary injury.40,41 There was a high rate of required interventions seen in this review, with 338 (31.5%) surgeries and 21 (2.0%) other invasive procedures. Risk factors associated with surgical intervention included abdominal pain, vomiting, fever, and handlebar sign, or “presence of a circular imprint on the body” (21.8% of injuries). In addition, accompanying thoracic injury may be associated with higher morbidity and need for operative intervention.40
Diagnostics
After initial examination and stabilization, further evaluation with laboratory tests and/or imaging should be considered judiciously to help guide downstream management. However, it is essential that the studies are tailored and guided by history, physical exam and the stability of the patient. As a general rule, a more severely injured patient will have more of a benefit from aggressive/invasive diagnostic modalities compared to the risk of multiple blood draws, exposure to radiation, and stress to the patient/family, with the inverse being true for more mildly injured patients. Deliberate consideration is recommended, and various clinical findings will need to be used jointly to decide next steps in care. Clinical decision tools have been developed to risk stratify patients for this purpose.42
Laboratory Tests
Laboratory evaluation is a useful adjunct in the workup of the unstable patient, and can be used in conjunction with other findings in the stable patient to potentially forgo CT imaging using certain decision rules.
There is no universal set of laboratory tests that is applicable for every patient, but it can be helpful to have information regarding acute blood loss anemia, acute kidney injury, hepatobiliary injury, coagulopathy, urinary tract injury, and hypoperfusion in the patient who can potentially decompensate. In the unstable blunt trauma patient, or those with penetrating trauma, it is reasonable to obtain a broad laboratory workup to include:3,43
- Complete blood count (CBC);
- Basic metabolic panel (BMP) and liver function tests (LFTs) together, or complete metabolic panel (CMP);
- Lipase;
- Type and screen;
- Coagulation studies (international normalized ratio [INR] and activated partial thromboplastin time [aPTT]);
- Urinalysis (UA);
- Toxicology screens in the appropriate setting;
- Pregnancy test (if of child-bearing age);
- Lactate;
- Venous blood gas (VBG).
If a child presents with isolated blunt abdominal trauma and is stable, a more sparing approach to laboratory tests should be considered, with the caveat that interpretation should be done with caution and used alongside other clinical findings, since their utility in isolation is limited. Even liver enzymes and UA, which have classically been described as indicators to evaluate for IAI are insufficient in predicting IAI when used alone.44-46
Aspartate aminotransferase (AST, also known as serum glutamic-oxaloacetic transaminase, or SGOT) and alanine transaminase (ALT, also known as serum glutamic-pyruvic transaminase, or SGPT) tend to elevate rapidly with hepatic injury, with levels of AST > 200 U/L or ALT > 125 U/L classically used as cutoffs to identify potential significant IAI.4,47-49 However, it is not sufficiently sensitive on its own to exclude pathology, according to a recent study by Kuas et al.50 A retrospective study by Otaibi et al found that AST was an independent predictor of IAI, but that among those with injury, only 37.3% of patients had abnormal lab results, highlighting the importance of using them in conjunction with other factors.46
Gross hematuria often correlates with IAI and should prompt further imaging workup, including retrograde cystography if stable and a bladder injury is suspected (i.e., due to associated pelvic fracture).4,44,45,49 However, it is unclear what cutoff should be used to define microhematuria and its clinical significance; some sources claim a cutoff of > 5 red blood cells/high-powered field (RBC/HPF) as correlating with IAI, while other sources recommend only using it in the context of other high-risk findings.4,51-52
Imaging
Similar to the use of laboratory tests, imaging should be used judiciously with consideration of risks and benefits of each modality, keeping in mind the differing test characteristics of each.
X-Ray
While plain radiographs of the chest and pelvis are routinely completed in most adult trauma cases, they should be used more judiciously in the pediatric population. If there is concern for accompanying thoracic trauma, a prompt chest X-ray should be obtained because many pediatric chest injuries requiring intervention may be diagnosed by them.3,43 If a patient is hemodynamically stable without pelvic pain or tenderness on exam, the use of routine pelvic X-ray can be deferred.6,43,44,53-55 However, if the patient is hemodynamically unstable, or has pelvic instability, hematuria, a decreased level of consciousness, or obvious femur deformity, a pelvic X-ray should be considered as a first test.4,43,53 X-ray alone should not be used to rule out pelvic fracture if negative with high clinical suspicion, since sensitivity ranges from 50% to 80% in pediatric patients.4,43,53,56 Because pelvic fractures in pediatrics are highly associated with other injuries, CT of the pelvis should be completed if fracture is strongly suspected, even with a negative X-ray.4,30
Ultrasound
Based on a recent consensus statement, landmarks that should be included in the FAST exam are the bilateral supra/subdiaphragmatic spaces, hepatorenal recess, caudal edge of the liver, inferior/superior pole of the kidney; spleen, splenorenal recess, spleen tip; bladder, rectouterine/rectovesical space; hepatopericardial interface, anterior/posterior/periapical border, and right ventricle (RV) and left ventricle (LV).57 The “extended” portion of the exam includes lung views to evaluate for pneumothorax. It is important to consider that one of the limitations of any point-of-care ultrasound is operator-dependence. A recent retrospective cohort study showed none of the 200 FAST scans selected for review showed all 30 expected landmarks.58 (See Figure 1.)
The FAST exam has a relatively low sensitivity and high specificity, which has been well studied and integrated into the trauma bay workflow in adult traumas. It has similar test characteristics in pediatrics, with a recent systematic review/meta-analysis showing a pooled sensitivity of 35% and specificity of 96% in pediatric blunt trauma.27 Long et al found that the FAST exam was specific in predicting early surgical intervention in pediatric patients, particularly if they were unstable in triage.59 Thus, it functions as a rule-in test, but it cannot be used as a rule-out test, meaning that if there is a positive FAST exam, intraperitoneal free fluid is likely, but if none is seen, it does not rule out IAI. In the unstable patient, the FAST exam can rapidly identify hemorrhage in a compartment (pericardial, intraperitoneal, or pleural) that may direct operative management.
Figure 1. Landmarks Visualized by FAST Exam |
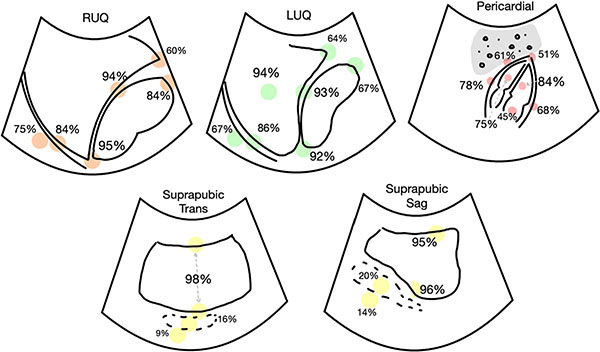 |
Image used with permission from: Firnberg M, Addo N, Lin-Martore M, et al. Evaluation of focused assessment with sonography for trauma completeness of children in the clinical setting. J Ultrasound Med. 2024;43:873-879. |
The role in a more stable patient is more controversial.60 The most recent ATLS guidelines propose that the FAST exam should not be used in isolation to rule out the presence of an IAI, but that if a patient is stable and free fluid is seen, a follow-up CT should be obtained.3
The major benefits of the FAST exam is that it is noninvasive, low cost, quick, and requires no radiation, acting almost as an extension of the abdominal exam. For instance, a retrospective study by Kornblith et al found that both the physical exam and FAST were independent predictors of IAI, but they performed better in combination than either did alone.61 It also may play a role in serial examinations throughout resuscitation, since it easily can be repeated if there are hemodynamic or physical exam changes. A small pilot study evaluating serial FAST exams in patients with initially negative scans suggests an improved sensitivity of 66.7%.62
Contrast-Enhanced Ultrasound
The use of contrast agents in ultrasound has been found to increase the detection rate of clinically significant injuries. Contrast agents consist of lipophilic compounds that envelop inert gases, which are injected intravenously and highlight the capillary vasculature, making extravasation visible.63 They are safe for use in patients with hepatic and renal failure, but no studies have been performed to date on their safety in pregnant patients.63,64 The test can be performed quickly at the bedside, especially for patients who are not stable enough to be transported to the CT scanner.
Jannatdoust et al and Pegoraro et al both recently performed meta-analyses of contrast-enhanced ultrasound (CEUS) performed by radiologists on pediatric blunt abdominal trauma. Pegoraro et al suggested sensitivity and specificity of CEUS ranged from 85.7% to 100% and 89% to 100%, respectively, while Jannatdoust suggested a pooled sensitivity of 88.5% and specificity of 98.5%, with an area under the curve of 96%.65,66 They both agree that the improved sensitivity compared to a regular FAST exam makes CEUS safe and accurate in recognizing clinically significant IAI. A recent study by Donner et al suggests that emergency providers may be able to perform the exam in adults with excellent test characteristics as well.67
While it is promising in terms of detection rate, it does have barriers to adoption in terms of requiring specific software to run the scans with a low mechanical index (to avoid prematurely popping the gas bubbles in the contrast agent), as well as training scanners on how to perform the study.63
Computed Tomography
CT with IV contrast is considered the gold standard for diagnosis of IAI in hemodynamically stable pediatric trauma patients.4,6,44,53 CT can be reliably used to detect intra-abdominal solid organ injury in pediatric patients with a sensitivity often found to be > 95%.4,42,53 Mesenteric and hollow viscus injuries, although rare, are harder to find; some studies suggest a high sensitivity in the 90s, but others suggest values as low as 17% to 50%.6,42,68,69 If hollow viscus injury is strongly suspected despite a negative CT, a repeat scan with oral contrast is not recommended, because it does not show increased diagnostic accuracy.42,53 Patients are better served with early surgical consultation and serial abdominal exams.4,43,44,53
CT scanning holds a risk of radiation-induced malignancy, particularly in pediatric patients.4,44,70,71 The overall risk is estimated at one in 300 to 600, with increased risk with younger age at scan, leading to increased prevalence of hematologic and intracranial cancers. The amount of radiation also tends to vary between pediatric and non-pediatric institutions.72-81 Steps are being taken to minimize the amount of radiation used in pediatric scan protocols, in keeping with As Low As Reasonably Achievable (ALARA) principles; clinical decision guidelines also have been formulated to avoid use of CT scanning in very low risk, stable patients.68,82,83
Clinical Decision Rules
In 2013, Holmes et al in the Pediatric Emergency Care Applied Research Network (PECARN) derived a prediction rule, using solely history and physical exam findings, that would allow for distinguishing the pediatric patients with blunt torso trauma who would be at very low risk for clinically significant IAI, which was defined as an injury requiring acute intervention.26 In this prospective observational study, their derived prediction rule included the following variables: evidence of abdominal wall trauma (including seatbelt sign), GCS < 14, abdominal tenderness on exam, thoracic wall trauma, complaints of abdominal pain, decreased breath sounds, and vomiting. Using this rule, the patients without any of these variables present were at very low risk (0.1%) of IAI requiring intervention. Their sensitivity and negative predictive value were 97% and 99.9%, respectively.26 (See Table 1.)
Table 1. Risk for Clinically Significant Intra-Abdominal Injury |
Very Low Risk
Low-to-Intermediate Risk
High Risk
|
PECARN: Pediatric Emergency Care Applied Research Network; GCS: Glasgow Coma Scale; CT: computed tomography; IAI: intra-abdominal injury Adapted from: Holmes JF, Lillis K, Monroe D, et al. Identifying children at very low risk of clinically important blunt abdominal injuries. Ann Emerg Med. 2013;62(2):107-116.e2. |
Since its initial publication, the PECARN rule has been externally validated in multiple studies.26,84,85 Springer et al conducted a single institution retrospective review, finding that one of 133 included patients with clinically significant IAI would have been identified as low risk, with a sensitivity of 99%.86 Kornblith et al found this PECARN algorithm to be highly predictive across two datasets.87 A retrospective review by Caylak et al compared the PECARN clinical decision rules to clinician suspicion and found that they were statistically similar in detection of injury, but that the use of the PECARN rule jointly with clinical judgment would decrease unnecessary CT scans.88
Holmes et al completed a multicenter prospective validation study of the rule and found that it performed well, with a sensitivity of 100% and negative predictive value of 100%.89 Additionally, Sigal et al also completed an external validation for the PECARN rule in a non-pediatric hospital and found the rule to have a sensitivity and negative predictive value (NPV) of 91.3% and 99.5%, respectively.84 Taken together, these results support the implementation of this clinical decision rule to aid in recognizing patients that are low risk for IAI and reducing unneeded CT scan use. However, the PECARN algorithm does not mean that any patient with a “yes” response necessitates a CT scan, since this likely would increase its use. Instead, it should be used as an additional tool in risk stratifying those patients.26,89
Several other clinical decision rules also have been developed to aid in identifying patients who were low risk for IAI. In 2009, a retrospective study by Karam et al led to the creation of the Blunt Abdominal Trauma in Children (BATiC) score that calculated points using the following variables: abnormal ultrasound findings, abdominal pain, peritoneal irritation, hemodynamic instability and several laboratory tests, including AST, ALT, WBC, lactate dehydrogenase (LDH), lipase, and creatinine. The researchers suggested that a BATiC score of ≤ 7 correlated to a low probability of IAI, with an NPV of 97%.90 An external validation study was done by de Jong et al that demonstrated similar test characteristics.91 While these findings were promising, further validation is lacking; critics have pointed toward its limited reliability and missing data, resulting in this score not being widely used.92
Streck et al also prospectively created a prediction rule to identify pediatric patients at low risk for IAI after blunt abdominal injury, with values that included AST > 200 U/L, abnormal abdominal exam, abnormal chest X-ray, report of abdominal pain, and abnormal pancreatic enzymes. This rule was found to have an NPV of 99.4% for IAI when none of the variables were present.93,94 This was externally validated in 2018 by Arbra et al, with similar findings of a sensitivity of 97.5% and an NPV of 99.3%.95 Recently, Ozcan and colleagues retrospectively compared these three clinical prediction rules and showed that both the PECARN and Streck rules have a high NPV to detect patients who are low risk for IAI, with the PECARN rules having the benefit of not requiring any laboratory tests or imaging to use.96
Emergency Department Management
Once a patient has been identified as high risk or positive for IAI, either in the trauma bay or after laboratory/imaging workup, treatment and disposition should be dictated by the severity of disease, as defined by hemodynamic stability and response to attempted stabilization. Transient or insufficient response to initial volume support should prompt consideration of more invasive management.
Volume Expansion
Hemorrhagic shock should be treated with early transfusion. A prospective observational study by Polites et al found that increased crystalloid use was associated with prolonged ventilation, and ICU and hospital stay; it did not decrease transfusion; and time to transfuse was associated with negative outcomes.97 A retrospective cohort study of the TQIP database by Akl et al found that a higher fresh frozen plasma (FFP):packed red blood cell (PRBC) ratio led to improved outcomes, including 24-hour survival, in-hospital mortality, and decreased need for PRBC transfusion volume.98 Butler et al also performed an analysis of TQIP finding similar results showing improved in-hospital mortality with increased FFP:PRBC ratios, without improved outcome with platelet:PRBC ratios (with the limitation that it may have been underpowered for this result).33
Whole blood is recommended if available, since it ”may lead to less dilutional coagulopathy, less hypocalcemia, and receipt of fewer blood products overall.”32 A recent observational study at UPMC Children’s Hospital of Pittsburgh of pediatric patients between 2013 and 2020 receiving massive transfusion (> 40 mL/kg) suggests a 72-hour mortality benefit with whole blood; in addition, they received less blood product overall.99
Resuscitative Endovascular Balloon Occlusion of the Aorta
Resuscitative endovascular balloon occlusion of the aorta (REBOA) has been studied in adult trauma, with one meta-analysis suggesting mortality benefit after a sensitivity analysis.100 Evidence is sparse in pediatrics, with one systematic review finding only one retrospective study and no trials.101 Smaller catheters are becoming more available; further study is needed to identify possible indications and risks/benefits of the procedure. This intervention may be considered as a temporizing measure in the situation where an OR is not immediately available for treatment of an unstable trauma patient with known bleeding below the diaphragm.
Antifibrinolytics
Data are mixed regarding the effectiveness of TXA, with one trial suggesting improved six-hour mortality.32 The PTHSCC recommends consideration of administering TXA within three hours of trauma, but with very low certainty of evidence.31 They do not recommend empirically administering PCC, cryoprecipitate, or fibrinogen (although if fibrinogen is < 150 mg/dL, it should be repleted).
Pain Control
Pain assessment in pediatric patients can be difficult, especially in nonverbal or younger patients. Validated pain assessment mechanisms, such as the revised Faces, Legs, Activity, Cry, Consolability (r-FLACC) in patients < 3 years of age or with cognitive impairment, Faces Pain Scale-Revised (FPS-R) in patients 3-8 years of age, and numerical pain scores in patients 8 years of age and older, should be used.102
To help minimize anxiety and alleviate pain, nonpharmacologic treatment should be given alongside medications. These can include parental presence in all age groups (with skin-to-skin contact or breast feeding for infants), sucrose water in the infant age group, and distraction or child life therapy in older age groups.102
In pediatric patients with ready IV access, opioid pain medications such as fentanyl (1 mcg/kg to 2 mcg/kg) can be given for rapid analgesia in the trauma bay.103 Patients who are requiring analgesia without ready access and who are intolerant of oral intake can be given intranasal fentanyl at 1.5 mcg/kg to 2.0 mcg/kg for rapid pain control. Subdissociative ketamine given intranasally at 0.5 mg/kg to 1.0 mg/kg also can be effective for this indication.103 Antiemetics, such as ondansetron at 0.1 mg/kg to 0.15 mg/kg, can be considered acutely as either an IV or oral dissolving tablet for the acute treatment of nausea.
Disposition Considerations and Definitive Management
Nonoperative Management
Not all patients with IAI found on imaging require operative management. Nonoperative management may include observation with serial abdominal exams and laboratory tests or interventional radiologic procedures, such as angioembolization (AE). Of patients with solid organ injury who do not meet immediate criteria for emergent operative intervention, most may be managed nonoperatively (up to 96.5% per a recent review).104 The American Pediatric Surgical Association (APSA) recommends risk stratification based on physiologic status, not injury grade. Operative management should be considered in any patient who is a “non-responder,” defined as “recurrent hypotension due to lack of sustained response to transfusion using MTP [massive transfusion] protocol; require > 40 mL/kg of blood products and/or those who have received greater than 4 units of pRBC without hemodynamic stabilization; or recurrent hemorrhage occurring later in the course.”105 APSA also recommends that all patients who are transient responders or non-responders should be admitted to the ICU, with all others recommended to be admitted to the hospital floor for observation.105 Guidelines from the Children’s Hospital of Philadelphia (CHOP) follow similar lines, also incorporating hemoglobin < 7 g/dL and injury grade V as indications for ICU admission (https://pathways.chop.edu/clinical-pathway/abdominal-injury-blunt-solid-organ-clinical-pathway).47
Per APSA, patients managed in the ICU should have repeat hemoglobin or hematocrit measurements every six hours, while floor patients should have these laboratory tests performed with a frequency dictated by their vital signs. They should have nothing by mouth and be restricted to bed rest until their vital signs normalize.105 Transfusion goals include:
- packed red blood cells (pRBC) to goal of > 7;
- FFP to INR < 2.0 if history of bleeding or high risk of rebleeding;
- Platelets to > 50,000 if history of bleeding or high risk of rebleeding; > 20,000 for others.105
Failure of Nonoperative Management
A small percentage of patients initially undergoing nonoperative management ultimately will “fail,” or require operative care during their admission. According to a recent secondary analysis of the Japanese SHIPPs (Splenic and Hepatic Injury in Pediatric Patients) study, nonoperative management failure occurred about 0.8% of the time. Factors suggesting failure of nonoperative management included “older age, positive FAST, contrast extravasation on CT, severe liver injury, concomitant pancreas injury, concomitant gastrointestinal injury, concomitant mesenteric injury, and higher Injury Severity Score.”106 For this reason, it is important that surgeons manage these trauma patients with IAI, even if it is anticipated that they ultimately will not require surgery. If a pediatric surgeon is not available at the facility where the patient initially presents with IAI on imaging, the patient’s case should be discussed with a surgeon at a trauma center for possible transfer.
Angiography and Embolization
The use of interventional radiology (IR) procedures to embolize bleeding blood vessels has become more popular in recent years in pediatric trauma. APSA, in its latest guidelines, examines evidence showing improved rates of splenic salvage in adults undergoing AE, but with no corresponding improvement in pediatric outcomes. APSA recommends angiography for patients with hepatic or splenic injuries as dictated by clinical status (ongoing bleeding despite transfusion and stable for travel to the IR suite), and recommends against prophylactic angiography in asymptomatic patients with contrast blush.105 Angioembolization should be considered over operative management in high-grade (III-V) renal injury with hemodynamic stability, especially if radiographic indicators, such as “hematoma rim distance greater than 3.5 cm, intravascular contrast extravasation, and medial renal laceration site,” are present, due to increased risk of renal loss.107
Considerations for Specific Organ Injuries
Spleen
Vaccinations should be administered if splenectomy is required, because of the risk of overwhelming post-splenectomy infection (OPSI). Although it has become less frequent, mortality can reach as high as 50%.104 The vaccines include Streptococcus pneumoniae, Haemophilus influenzae (type b), and Neisseria meningitidis, as well as annual vaccination against influenza.104 OPSI can present with prodromal nonspecific flu-like symptoms and progress to septic shock, coma, and disseminated intravascular coagulopathy. All post-splenectomy patients should be educated to inform all future providers of their immunocompromised status and should be carefully evaluated if presenting in the future with signs of infection.108
Renal and Urinary System Injuries
The Eastern Association for the Surgery of Trauma (EAST) recommends nonoperative over operative management for hemodynamically stable patients for kidney injury, because of the increased risk of renal loss and need for blood transfusion in the available studies. Similar to other solid organs, the only absolute indication for surgical intervention is non-response to volume resuscitation.107 Angioembolization is favored over operative management for isolated high-grade renal injuries. Follow-up imaging may be performed with CEUS, since it has the added advantage of assessing the collecting duct.63 Posttraumatic renal hypertension should be monitored after the injury with blood pressure checks.107
Ureteral injury is rare in pediatrics (< 1% of abdominal trauma). Perirenal stranding/hematoma or low-density retroperitoneal fluid can suggest this diagnosis on the CT scan, and the confirmatory test is a retrograde pyelogram. Surgical treatment can involve ureteral stenting alone for low-grade injuries, or percutaneous nephrostomy tube placement or ureteroureterostomy or ureteral reimplantation.109
Bladder injuries usually are associated with concomitant pelvic fractures because of the associated high-energy mechanism. If this is confirmed by retrograde cystography, isolated bladder rupture limited to the extraperitoneum can be managed with transurethral catheter drainage and antibiotics, but surgical repair is indicated for persistent extravasation, concomitant vaginal or rectal injuries, bladder neck lesions, and patients undergoing laparotomy for other injuries or internal fixation of pelvic fracture.109
Urethral injury is more common in males and should be suspected when gross blood is present at the meatus, especially in the setting of polytrauma; after confirmation with a retrograde urethrogram, it can be treated with a suprapubic catheter initially, followed by either transurethral catheter placement (for anterior injuries) or surgical repair (for posterior injuries).35
Pancreaticoduodenal Injury
Pediatric duodenal and pancreatic injury are rare but can have long-reaching sequelae (such as fistulae or pseudocysts). This is classically associated with a delayed presentation, especially after handlebar injury. Initial CT has sensitivity and specificity of 86% and 88%, respectively, for duodenal injury; concerning signs include symptoms of bowel obstruction (duodenal) or elevated or rising amylase/lipase 12-24 hours after the injury (pancreatic) and should prompt repeat imaging, such as a CT with specific pancreatic phase.40,41 Immediate operative management is indicated for any unstable patient despite volume expansion, any evisceration, impalement, or sign of peritonitis; nonoperative management can be considered in the stable patient with low-grade injury.41 For ultimate disposition, surgical associations recommend management of these injuries at a pediatric trauma center, since certain lesions may require pancreaticoduodenectomy (Whipple procedure) using damage control techniques and staged reconstruction, preferably by an experienced surgeon.41
Extrahepatic Biliary Tree Injury
This injury pattern is rare, but may require cholecystectomy, hepaticojejunostomy, or choledochojejunostomy for ultimate repair. If there are concerning signs of damage to the gallbladder or biliary tree during exploratory laparotomy, an intraoperative cholangiogram can be performed; whereas if it is suspected in a patient currently undergoing nonoperative management, a magnetic resonance cholangiopancreatography (MRCP) scan is preferred for definitive diagnosis with hepatobiliary contrast agents added to evaluate for bile leaks.41 Hepatobiliary scintigraphy with a hepatobiliary iminodiacetic acid (HIDA) scan is not indicated due to the long scan time, making it not useful in the trauma setting.41
Special Considerations
Management of Penetrating Trauma
Unlike blunt trauma, there are no specific recommendations in the pediatric realm regarding nonoperative management in the setting of penetrating trauma. Patients with signs of peritonitis, diffuse tenderness, or hemodynamic instability despite resuscitation should go to the OR. Local wound exploration may be performed at the bedside by the surgeon in a stable patient. If the wound does not penetrate the anterior fascia, EAST recommendations suggest that in adults, the subset of stab wound patients who are hemodynamically stable without signs of peritonitis or diffuse abdominal tenderness, or gunshot patients with tangential wounds and without peritoneal signs, may instead have an initial CT obtained followed by 24-hour observation admission for serial abdominal exams, with decision-making performed in conjunction with the consulting surgeon.36 This appears to be a reasonable approach in pediatrics as well.
Evaluation and Management of Nonaccidental Trauma
Clinical features concerning for nonaccidental trauma (NAT) include multiple injuries (especially in multiple stages of healing), delay in seeking care, mechanism not concordant with level of development, and/or implausible injury for mechanism.110 Notably, studies have shown that the majority of falls, a commonly reported cause of pediatric trauma, do not result in significant IAI.110 Consequently, if a patient is found to have IAI, but this does not match with the history of a fall, NAT should be considered. Abdominal exam findings are less sensitive for IAI in victims of abuse. Hollow viscus and pancreatic injuries are more common than in the general population, but solid organ injuries still are the most common. No single IAI is pathognomonic for NAT — any organ can be injured in abusive trauma. Finding one injury concerning for NAT should prompt investigation for injuries in other organ systems. Injury severity and mortality rate are higher in patients with NAT than with accidental trauma.3
Use caution with applying clinical decision tools, such as PECARN, to suspected abuse because they may present outside the 24-hour time window or may not have a clear history of trauma. Applying these rules indiscriminately could miss important injuries that fall outside of PECARN inclusion criteria.110
Laboratory tests should include LFTs, lipase, and UA for risk stratification, and it is recommended to complete a contrast-enhanced CT in any patients with concern for NAT with any signs/symptoms on the abdomen or an AST/ALT > 80.5 CEUS is emerging as a promising radiation-free alternative, but it needs further research to validate its use in the abuse setting.110
EPs must familiarize themselves with reporting requirements for NAT, since in many jurisdictions they are required to disclose these episodes to local social service agencies or health and human services departments even if abuse is only suspected. Thirty-three percent of patients who ultimately die from assault in the United States or United Kingdom were previously victims of maltreatment.3
Conclusion
Because of their anatomical and physiological variances, pediatric trauma patients can lead to unique challenges in the ED. However, it is imperative for EPs to be prepared to treat trauma patients of any age in any setting, particularly since trauma remains the leading cause of morbidity and mortality in the pediatric population. With any trauma patient, it is important to follow a step-wise approach, such as using the ATLS, to ensure that the most emergent life threats are being addressed first before moving on to further diagnostics and definitive care. In the setting of suspected pediatric abdominal trauma, important information to obtain includes mechanism of injury, vital signs, and current symptoms. A thorough abdominal exam including both palpation and visual inspection should be completed. An eFAST exam should be completed alongside this initial evaluation, since a positive exam may lead to prompt operative care in the unstable patient.
In the stable patient, judicious thought should be given to further diagnostics and the risks vs. benefits should be weighed on a case-by-case basis. Laboratory tests to consider based on a given scenario include CBC, BMP, LFTs, lipase, type and screen, UA, coagulation studies, pregnancy test, toxicology screens, lactate, and VBG. Risk stratification tools, such as the validated PECARN algorithm, should be used when deciding which patients warrant further workup with a CT scan.
Patients with identified IAI should be managed in consultation with a pediatric surgeon. It is recommended that pediatric patients with IAI are stratified on physiologic status rather than injury severity and, if hemodynamically stable or responsive to resuscitative measures, many patients will be able to be managed nonoperatively.
Jordan Hickey, MD, is Instructor, Core Faculty Member, Department of Emergency Medicine, Wright State University Boonshoft School of Medicine, Dayton, OH.
Charles Jang, MD, is Resident, Wright-Patterson Air Force Base/Wright State University Boonshoft School of Medicine, Dayton, OH.
References
1. WISQARS Leading Causes of Death Visualization Tool. Centers for Disease Control and Prevention. https://wisqars.cdc.gov/lcd/
2. Mansuri F, Loux T, Brooks SE, et al. Temporal trends in patient characteristics, injury mechanisms and outcomes in pediatric trauma admissions between 2010 and 2017. Am J Surg. 2020;220(2):468-475.
3. Chapter 10: Pediatric Trauma. In: Advanced Trauma Life Support Student Course Manual. 10th ed. American College of Surgeons; 2018:186-212.
4. Singh S, Holmes J. Chapter 160: Pediatric Trauma. In: Bakes K, et al eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 10th ed. Elsevier; 2023:2052-2066.
5. Giovannini E, Santelli S, Pelletti G, et al. Pediatric motor vehicle crashes injuries: A systematic review for forensic evaluation. Int J Legal Med. 2024;138(4):1329-1341.
6. Vogel A, Dingeldein M. Chapter 120: Pediatric abdominal trauma. In: Zimmerman JJ, Clark RSB, Fuhrman BP, et al, eds. Fuhrman and Zimmerman’s Pediatric Critical Care. 6th Ed. Elsevier; 2022: 1408-1416.
7. WISQARS Fatal and Nonfatal Injury Reports. Centers for Disease Control and Prevention. https://wisqars.cdc.gov/reports/
8. WISQARS Fatal and Nonfatal Injury Reports. Centers for Disease Control and Prevention. https://wisqars.cdc.gov/reports/
9. Chaudhari PP, Rodean J, Spurrier RG, et al. Epidemiology and management of abdominal injuries in children. Acad Emerg Med. 2022;29(8):944-953.
10. Alvarez CA, Grigorian A, Swentek L, et al. Relationship of obesity and severe penetrating thoracic and abdominal injuries in adolescent patients. Am Surg. 2023;89(12):5744-5749.
11. Castelão M, Lopes G, Vieira M. Epidemiology of major paediatric trauma in a European Country — trends of a decade. BMC Pediatr. 2023;23:194.
12. Matsui M, Sugita K, Kawano T, et al. Cases of pediatric intra-abdominal solid organ injury induced by blunt trauma experienced over a 15-year period at two centers in Japan. World J Pediatr Surg. 2023;6(3):e000560.
13. Nimanya SA, Sekabira J, Kakembo N, et al. Pediatric abdominal trauma in a National Referral Hospital. Afr Health Sci. 2022;22(Spec Issue):108-113.
14. Molla YD, Mekonnen DC, Beza AD, et al. Surgical outcome of pediatric abdominal trauma at Tertiary Hospital, Northwest Ethiopia, a 3-year retrospective study. BMC Surg. 2024;24:203.
15. Divya G, Kundal VK, Addagatla R, et al. Spectrum of paediatric blunt abdominal trauma in a tertiary care hospital in India. Afr J Paediatr Surg AJPS. 2023;20(3):191.
16. Alomani H, Fareed A, Ibrahim H, et al. Pediatric trauma at a single center in the Qassim region of Saudi Arabia. Ann Saudi Med. 2021;41(3):165-170.
17. Alattar Z, Hoebee S, Ron E, et al. The role of obesity in motor vehicle injuries and fatalities in the pediatric population: A systematic review. J Intensive Care Med. 2022;37(4):472-479.
18. Mulvihill H, Roster K, Lakhi N. Obesity as a risk factor for adverse outcomes after pedestrian trauma accidents in the pediatric population. Pediatr Emerg Care. 2024;40(7):498-503.
19. Castle SL, Barthel ER, Tamura DY. Obesity protects against operation in pediatric penetrating trauma to the torso. J Surg Res. 2021;263:57-62.
20. Cicero MX, Adelgais K, Funaro MC, et al. Prehospital trauma compendium: Pediatric severe and inflicted trauma — A position statement and resource document of NAEMSP. Prehosp Emerg Care. 2025; Feb 10:1-11. [Online ahead of print].
21. Cercone A, Ramgopal S, Martin-Gill C. Completeness of pediatric versus adult patient assessment documentation in the National Emergency Medical Services Information System. Prehosp Emerg Care. 2024;28(2):243-252.
22. Newgard CD, Fischer PE, Gestring M, et al. National guideline for the field triage of injured patients: Recommendations of the National Expert Panel on Field Triage, 2021. J Trauma Acute Care Surg. 2022;93(2):e49-e60.
23. Acker SN, Ross JT, Partrick DA, et al. Pediatric specific shock index accurately identifies severely injured children. J Pediatr Surg. 2015;50(2):331-334.
24. Snyder KB, Phillips R, Stewart K, et al. SIPA poorly predicts outcomes in young pediatric trauma patients. J Pediatr Surg. 2025;60(1):161997.
25. Yoon SH, Shin SJ, Kim H, Roh YH. Shock index and shock index, pediatric age-adjusted as predictors of mortality in pediatric patients with trauma: A systematic review and meta-analysis. PLoS One. 2024;19(7):e0307367.
26. Holmes JF, Lillis K, Monroe D, et al. Identifying children at very low risk of clinically important blunt abdominal injuries. Ann Emerg Med. 2013;62(2):107-116.e2.
27. Liang T, Roseman E, Gao M, Sinert R. The utility of the focused assessment with sonography in trauma examination in pediatric blunt abdominal trauma: A systematic review and meta-analysis. Pediatr Emerg Care. 2021;37(2):108-118.
28. Murphy P, Leeper WR. Chapter 33: Trauma Ultrasound. In: Soni N, Arntfield R, Kory P, eds.Point-of-Care Ultrasound. 2nd ed. Elsevier. 2020: 310-322.e2.
29. Bašković M, Keretić D, Lacković M, et al. The diagnosis and management of pediatric blunt abdominal trauma — A comprehensive review. Diagnostics (Basel). 2024;14:2257.
30. Nguyen ATM, Drynan DP, Holland AJA. Paediatric pelvic fractures — an updated literature review. Anz J Surg. 2022;92(12):3182-3194.
31. Russell RT, Esparaz JR, Beckwith MA, et al. Pediatric traumatic hemorrhagic shock consensus conference recommendations. J Trauma Acute Care Surg. 2023;94(1):S2-S10.
32. Russell RT, Leeper CM, Spinella PC. Damage-control resuscitation in pediatric trauma: What you need to know. J Trauma Acute Care Surg. 2023;95(4):472-480.
33. Butler EK, Mills BM, Arbabi S, et al. Association of blood component ratios with 24-hour mortality in injured children receiving massive transfusion. Crit Care Med. 2019;47(7):975-983.
34. Abou Khalil E, Morgan KM, Gaines BA, et al. Use of whole blood in pediatric trauma: A narrative review. Trauma Surg Acute Care Open. 2024;9(Suppl 1):e001127.
35. Singer G, Arneitz C, Tschauner S, et al. Trauma in pediatric urology. Semin Pediatr Surg. 2021;30(4):151085.
36. Como JJ, Bokhari F, Chiu WC, et al. Practice management guidelines for selective nonoperative management of penetrating abdominal trauma. J Trauma Acute Care Surg. 2010;68(3):721.
37. Cico S, Caglar D. Chapter 155: Care of the Pediatric Patient. In: Bakes K, et al eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 10th ed. Elsevier;2023:1996-2004.
38. Halari MM, Charyk Stewart T, McClafferty KJ, et al. Injury patterns in motor vehicle collision-pediatric pedestrian deaths. Traffic Inj Prev. 2022;23(sup1):S68-S73.
39. Schubert A, Babisch S, Scanlon JM, et al. Passenger and heavy vehicle collisions with pedestrians: Assessment of injury mechanisms and risk. Accid Anal Prev. 2023;190:107139.
40. Cheung R, Shukla M, Akers KG, et al. Bicycle handlebar injuries — a systematic review of pediatric chest and abdominal injuries. Am J Emerg Med. 2022;51:13-21.
41. Coccolini F, Kobayashi L, Kluger Y, et al. Duodeno-pancreatic and extrahepatic biliary tree trauma: WSES-AAST guidelines. World J Emerg Surg WJES. 2019;14:56.
42. Achatz G, Schwabe K, Brill S, et al. Diagnostic options for blunt abdominal trauma. Eur J Trauma Emerg Surg. 2022;48(5):3575-3589.
43. Guyther J, Wiltjer R. Pediatric trauma. Emerg Med Clin North Am. 2023;41(1):205-222.
44. Ernst G. Chapter 110: Pediatric Trauma. In: Tintinalli et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 9th ed. McGraw-Hill;2019:689-698.
45. Alzahem AM, Soundappan SSV, Cass DT. The predictors for positive yield abdominal computed tomography in pediatric abdominal trauma. Pediatr Emerg Care. 2020;36(10):e543-e548.
46. Otaibi BW, Khurshid MH, Hejazi O, et al. The abdomen does not lie, but the labs might: Predictors of intra-abdominal injury on computed tomography imaging in pediatric blunt trauma patients. J Trauma Acute Care Surg. 2025; Jan 6. doi: 10.1097/TA.0000000000004549. [Online ahead of print].
47. Children’s Hospital of Philadelphia. Perks D, Nance M, Osipowicz J. Emergency Department, Inpatient, and ICU Clinical Pathway for Children with Blunt Abdominal Solid Organ Injury. Last revised March 2025. https://pathways.chop.edu/clinical-pathway/abdominal-injury-blunt-solid-organ-clinical-pathway
48. Gosztyla C, Walk R. Liver Injury. In: Kennedy AP, Ignacio RC, Ricca R, eds. Pediatric Trauma Care. Springer International Publishing;2022:269-278.
49. Weber B, Lackner I, Braun CK, et al. Laboratory markers in the management of pediatric polytrauma: Current role and areas of future research. Front Pediatr. 2021;9:622753.
50. Kuas C, Acar N, Ozakin E, et al. The diagnostic value of laboratory tests in detecting solid organ injuries in pediatric patients with blunt abdominal trauma. Am J Emerg Med. 2022;57:133-137.
51. Casson C, Jones RE, Gee KM, Beres AL. Does microscopic hematuria after pediatric blunt trauma indicate clinically significant injury? J Surg Res. 2019;241:317-322.
52. Papillon SC, Pennell CP, Bauer SE, et al. Presence of microscopic hematuria does not predict clinically important intra-abdominal injury in children. Pediatr Emerg Care. 2024;40(8):e139-e142.
53. Joshi A, Hamman SM, Corbitt NM. Imaging of pediatric blunt abdominal trauma. In: Otero HJ, Kaplan SL, Medina LS, et al, eds. Evidence-Based Imaging in Pediatrics: Clinical Decision Support for Optimized Imaging in Pediatric Care. Springer International Publishing;2024:751-771.
54. Haasz M, Simone LA, Wales PW, et al. Which pediatric blunt trauma patients do not require pelvic imaging? J Trauma Acute Care Surg. 2015;79(5):828-832.
55. Lagisetty J, Slovis T, Thomas R, et al. Are routine pelvic radiographs in major pediatric blunt trauma necessary? Pediatr Radiol. 2012;42(7):853-858.
56. Kwok MY, Yen K, Atabaki S, et al. Sensitivity of plain pelvis radiography in children with blunt torso trauma. Ann Emerg Med. 2015;65(1):63-71.e1.
57. Kornblith AE, Addo N, Plasencia M, et al. Development of a consensus-based definition of focused assessment with sonography for trauma in children. JAMA Netw Open. 2022;5(3):e222922.
58. Firnberg M, Addo N, Lin-Martore M, et al. Evaluation of focused assessment with sonography for trauma completeness of children in the clinical setting. J Ultrasound Med. 2024;43(5):873-879.
59. Long MK, Vohra MK, Bonnette A, et al. Focused assessment with sonography for trauma in predicting early surgical intervention in hemodynamically unstable children with blunt abdominal trauma. J Am Coll Emerg Physicians Open. 2022;3(1):e12650.
60. Lee MS, Sweetnam-Holmes D, Soffer GP, Harel-Sterling M. Updates on the clinical integration of point-of-care ultrasound in pediatric emergency medicine. Curr Opin Pediatr. 2024;36(3):256.
61. Kornblith AE, Graf J, Addo N, et al. The utility of focused assessment with sonography for trauma enhanced physical examination in children with blunt torso trauma. Acad Emerg Med. 2020;27(9):866-875.
62. Nti BK, Benzoni N, Starr R, et al. Serial Trauma Abdominal Ultrasound in Children (STAUNCH): A pilot study. Pediatr Emerg Care. 2024;40(9):623-626.
63. Squires JH, McCarville MB. Contrast-enhanced ultrasound in children: Implementation and key diagnostic applications. AJR Am J Roentgenol. 2021;217(5):1217-1231.
64. Muskula PR, Main ML. Safety with echocardiographic contrast agents. Circ Cardiovasc Imaging. 2017;10(4):e005459.
65. Jannatdoust P, Valizadeh P, Hassankhani A, et al. Diagnostic performance of contrast-enhanced ultrasound in traumatic solid organ injuries in children: A systematic review and meta-analysis. Pediatr Radiol. 2024;55(2):226-241.
66. Pegoraro F, Giusti G, Giacalone M, Parri N. Contrast-enhanced ultrasound in pediatric blunt abdominal trauma: A systematic review. J Ultrasound. 2022;25(3):419-427.
67. Donner V, Thaler J, Hautz WE, et al. Contrast-enhanced point of care ultrasound for the evaluation of stable blunt abdominal trauma by the emergency physician: A prospective diagnostic study. J Am Coll Emerg Physicians Open. 2024;5(2):e13123.
68. Scott J, Grewal T, Brewster S, Khan A. Optimizing imaging in the pediatric trauma patient, part 2: Thoracic and abdominal trauma. Pediatr Emerg Med Pract. 2022;19(Suppl 9):1-26.
69. Staab V, Naganathan S, McGuire M, et al. Gastrointestinal perforation with blunt abdominal trauma in children. Children. 2024;11(6):612.
70. Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176(2):289-296.
71. National Cancer Institute. Radiation Risks and Pediatric Computed Tomography (CT): A Guide for Health Care Providers. Last reviewed Sept. 4, 2018. https://www.cancer.gov/about-cancer/causes-prevention/risk/radiation/pediatric-ct-scans
72. Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet. 2012;380(9840):499-505.
73. Li IG, Yang YH, Li YT, Tsai YH. Paediatric computed tomography and subsequent risk of leukaemia, intracranial malignancy and lymphoma: A nationwide population-based cohort study. Sci Rep. 2020;10(1):7759.
74. Bosch de Basea Gomez M, Thierry-Chef I, Harbron R, et al. Risk of hematological malignancies from CT radiation exposure in children, adolescents and young adults. Nat Med. 2023;29(12):3111-3119.
75. Meulepas JM, Ronckers CM, Smets AMJB, et al. Radiation exposure from pediatric CT scans and subsequent cancer risk in the Netherlands. J Natl Cancer Inst. 2019;111(3):256-263.
76. Wang WH, Sung CY, Wang SC, Shao YHJ. Risks of leukemia, intracranial tumours and lymphomas in childhood and early adulthood after pediatric radiation exposure from computed tomography. CMAJ. 2023;195(16):E575-E583.
77. Abalo KD, Rage E, Leuraud K, et al. Early life ionizing radiation exposure and cancer risks: Systematic review and meta-analysis. Pediatr Radiol. 2021;51(1):45-56.
78. Hrdy M, Mahesh M, Miller M, et al. An analysis of computed tomography-related radiation exposure in pediatric trauma patients. Pediatr Emerg Care. 2021;37(6):296.
79. Hassankhani A, Amoukhteh M, Jannatdoust P, et al. A systematic review and meta-analysis of incidental findings in computed tomography scans for pediatric trauma patients. Clin Imaging. 2023;103:109981.
80. Pariaszevski A, Wang NE, Lee MO, et al. Computed tomography rates in pediatric trauma patients among emergency medicine and pediatric emergency medicine physicians. J Pediatr Surg. 2023;58(2):315-319.
81. Strait L, Sussman R, Ata A, Edwards MJ. Utilization of CT imaging in minor pediatric head, thoracic, and abdominal trauma in the United States. J Pediatr Surg. 2020;55(9):1766-1772.
82. Sayed IS, Mohd Yusof MI. Techniques and strategies to minimize radiation exposure in pediatric computed tomography (CT) abdominal examinations: A review. Cureus. 2024;16(8):e67494.
83. Smith TB, Heil J, Frush DP, Samei E. Clinical concordance with Image Gently guidelines for pediatric computed tomography: A study across 663,417 CT scans at 53 clinical facilities. Pediatr Radiol. 2021;51(5):800-810.
84. Sigal AP, Deaner T, Woods S, et al. External validation of a pediatric decision rule for blunt abdominal trauma. J Am Coll Emerg Physicians Open. 2022;3(1):e12623.
85. Arnold CG, Ishimine P, McCarten-Gibbs KA, et al. Performance of individual criteria of the Pediatric Emergency Care Applied Research Network (PECARN) intraabdominal injury prediction rule. Acad Emerg Med. 2025; Jan 13. doi: 10.1111/acem.15084. [Online ahead of print].
86. Springer E, Frazier SB, Arnold DH, Vukovic AA. External validation of a clinical prediction rule for very low risk pediatric blunt abdominal trauma. Am J Emerg Med. 2019;37(9):1643-1648.
87. Kornblith AE, Singh C, Devlin G, et al. Predictability and stability testing to assess clinical decision instrument performance for children after blunt torso trauma. PLOS Digit Health. 2022;1(8):e0000076.
88. Çaylak ST, Yaka E, Yilmaz S, et al. Comparison of PECARN clinical decision rule and clinician suspicion in predicting intra-abdominal injury in children with blunt torso trauma in the emergency department. Turk J Trauma Emerg Surg. 2022;28(4):529.
89. Holmes JF, Yen K, Ugalde IT, et al. PECARN prediction rules for CT imaging of children presenting to the emergency department with blunt abdominal or minor head trauma: A multicentre prospective validation study. Lancet Child Adolesc Health. 2024;8(5):339-347.
90. Karam O, Sanchez O, Chardot C, La Scala G. Blunt abdominal trauma in children: A score to predict the absence of organ injury. J Pediatr. 2009;154(6):912-917.
91. de Jong WJJ, Stoepker L, Nellensteijn DR, et al. External validation of the Blunt Abdominal Trauma in Children (BATiC) score: Ruling out significant abdominal injury in children. J Trauma Acute Care Surg. 2014;76(5):1282-1287.
92. Russell WS. The BATiC score to rule out traumatic intra-abdominal injury. J Pediatr. 2014;165(5):1070-1071.
93. Streck CJ, Jewett BM, Wahlquist AH, et al. Evaluation for intra-abdominal injury in children after blunt torso trauma: Can we reduce unnecessary abdominal computed tomography by utilizing a clinical prediction model? J Trauma Acute Care Surg. 2012;73(2):371-376;
discussion 376.
94. Streck CJ, Vogel AM, Zhang J, et al. Identifying children at very low risk for blunt intra-abdominal injury in whom CT of the abdomen can be avoided safely. J Am Coll Surg. 2017;224(4):449-458.e3.
95. Arbra CA, Vogel AM, Plumblee L, et al. External validation of a five-variable clinical prediction rule for identifying children at very low risk for intra-abdominal injury after blunt abdominal trauma. J Trauma Acute Care Surg. 2018;85(1):71-77.
96. Ozcan A, Ahn T, Akay B, Menoch M. Imaging for pediatric blunt abdominal trauma with different prediction rules: Is the outcome the same? Pediatr Emerg Care. 2022;38(2):e654.
97. Polites SF, Moody S, Williams RF, et al. Timing and volume of crystalloid and blood products in pediatric trauma: An Eastern Association for the Surgery of Trauma multicenter prospective observational study. J Trauma Acute Care Surg. 2020;89(1):36.
98. Akl M, Anand T, Reina R, et al. Balanced hemostatic resuscitation for bleeding pediatric trauma patients: A nationwide quantitative analysis of outcomes. J Pediatr Surg. 2022;57(12):986-993.
99. Gaines BA, Yazer MH, Triulzi DJ, et al. Low titer group O whole blood In injured children requiring massive transfusion. Ann Surg. 2023;277(4):e919.
100. Granieri S, Frassini S, Cimbanassi S, et al. Impact of resuscitative endovascular balloon occlusion of the aorta (REBOA) in traumatic abdominal and pelvic exsanguination: A systematic review and meta-analysis. Eur J Trauma Emerg Surg. 2022;48(5):3561-3574.
101. Campagna GA, Cunningham ME, Hernandez JA, et al. The utility and promise of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in the pediatric population: An evidence-based review. J Pediatr Surg. 2020;55(10):2128-2133.
102. Shaikh H, Chumpitazi CE. Chapter 157: Pediatric Sedation and Analgesia. In: Walls R, Hockberger R, Gausche-Hill M, et al. Rosen’s Emergency Medicine: Concepts and Clinical Practice: 10th ed. 2023:2016-2029.e2.
103. Ali S, Poonai N. Chapter 115: Pain Management and Procedural Sedation for Infants and Children. In: Tintinalli JE, Ma OJ, Yealey D, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 9th ed. McGraw-Hill;2019:723-731.
104. Lyttle BD, Williams RF, Stylianos S. Management of pediatric solid organ injuries. Children. 2024;11(6):667.
105. Williams RF, Grewal H, Jamshidi R, et al. Updated APSA Guidelines for the Management of Blunt Liver and Spleen Injuries. J Pediatr Surg. 2023;58(8):1411-1418.
106. Nakao S, Katsura M, Yagi M, et al. Assessing associated factors for failure of nonoperative management in pediatric blunt liver and spleen injuries: A secondary analysis of the SHIPPs study. Eur J Trauma Emerg Surg. 2024;50(5):2249-2257.
107. Eastern Association for the Surgery of Trauma. Pediatric Blunt Renal Trauma. Published 2019. https://www.east.org/education-resources/practice-management-guidelines/details/pediatric-blunt-renal-trauma
108. Tahir F, Ahmed J, Malik F. Post-splenectomy sepsis: A review of the literature. Cureus. 12(2):e6898.
109. Singer G, Arneitz C, Tschauner S, et al. Trauma in pediatric urology. Semin Pediatr Surg. 2021;30(4):151085.
110. Henry MK, Bennett CE, Wood JN, Servaes S. Evaluation of the abdomen in the setting of suspected child abuse. Pediatr Radiol. 2021;51(6):1044-1050.