
Outpatient Management of Venous Thromboembolism Diagnosed in the ED
May 15, 2025
By Jessica Wanthal, MD; Megan Rivera, MD; William Osae, MD; and Sreeja Natesan, MD
EXECUTIVE SUMMARY
- Outpatient management of venous thromboembolism (VTE) diagnosed in the emergency department is feasible for selected patients.
- Direct oral anticoagulants (DOACs) like apixaban, rivaroxaban, and dabigatran are preferred for outpatient treatment because of their fixed dosing and lack of need for laboratory monitoring.
- Thrombolysis or interventional treatments in patients with VTE typically are reserved for hemodynamically unstable patients or those with limb-threatening deep vein thrombosis.
- Patients who may be candidates for outpatient therapy should have no contraindications to anticoagulation, such as recent bleeding, severe renal or liver disease, or severe thrombocytopenia.
- Patients with active cancer or severe renal failure may require alternative anticoagulants, such as low molecular weight heparin.
- Risk stratification tools, such as the Pulmonary Embolism Severity Index (PESI) and Hestia criteria, should be used to identify low-risk patients with pulmonary embolism (PE).
- Patients with PE who are hemodynamically stable, have no evidence of right ventricular strain, have normal cardiac biomarkers, and meet criteria for low-risk PE may be candidates for outpatient treatment.
- Patients suitable for outpatient management should have reliable outpatient follow-up and monitoring.
- The treatment duration of anticoagulation typically is a minimum of three months for VTE provoked by transient risk factors, while indefinite anticoagulation may be necessary for unprovoked VTE or VTE associated with persistent risk factors like cancer.
- Outpatient management can reduce healthcare costs and minimize the risk of hospital-acquired conditions.
Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is a commonly encountered and potentially life-threatening condition in emergency departments (EDs), affecting nearly 10 million people worldwide every year.1 In the United States, the incidence of PE is estimated at 23 to 69 cases per 100,000 individuals annually, with higher rates observed in elderly and hospitalized populations.2-4 PE is the third leading cause of cardiovascular death, following myocardial infarction and stroke, respectively, and accounts for 10% of all hospital deaths.3,5,6 Its presentation often is variable and nonspecific, ranging from mild dyspnea to pleuritic chest pain to sudden cardiac arrest, complicating timely diagnosis in the ED setting. The mortality ranges from 2% to as high as 30% in selected patient populations.3 Emerging evidence supports outpatient management for selected low-risk patients, offering an opportunity to reduce hospital admissions and enhance resource use.3 The use of validated risk stratification tools, along with the availability of direct oral anticoagulants (DOACs), has enabled a safe and effective shift toward outpatient care.1-4,7-9
Pathophysiology
DVT is the result of the triad of venous stasis, endothelial injury, and hypercoagulability. PE is caused by an embolic phenomenon affecting the pulmonary vasculature as the result of DVT, most commonly located in the lower extremities. The pulmonary vascular resistance is compromised due to the clot burden, placing strain on the right ventricle and leading to right heart failure and subsequently impaired filling of the left side of the heart. There is a ventilation-perfusion mismatch that occurs, resulting in reduced cardiac output, and it may precipitate shock or cardiovascular collapse depending on the location, clot burden, and cardiopulmonary reserve.1
History and Physical Exam
Patients presenting with DVTs often appear clinically well and have normal vital signs. Common symptoms suggestive of DVT include unilateral leg swelling, pain, and erythema that develop over several days.10 Homan’s sign is one traditional physical exam finding described as calf pain with dorsiflexion of the foot and historically has been suggestive of DVT, but it lacks sensitivity and specificity.11,12 As such, it has fallen out of favor in the clinical diagnosis of DVT. Importantly, pulses and compartments of all extremities should be assessed and documented in any patient for whom the clinician is suspicious of DVT. If a patient arrives with complaints of sudden onset severe pain and discoloration, this should raise concern for limb ischemia. This complication of DVT, called phlegmasia dolens, is a limb-threatening condition that warrants immediate recognition and intervention.
Patients with PE, on the other hand, can vary in their clinical presentation, from well-appearing with normal vital signs to overt shock and cardiopulmonary arrest. Common symptoms include tachycardia, shortness of breath, hypoxia, hemoptysis, and pleuritic chest pain. A thorough history should be obtained when VTE is suspected, focusing on symptom onset, history of malignancy, immobility or recent surgery, estrogen use, and any prior history of DVT or PE, or known thrombophilia.
Risk Factors
VTE is associated with both major and minor transient risk factors. The three mechanisms of DVT formation are venous stasis, endothelial injury, and hypercoagulability. Table 1 shows the risk factors related to each of these mechanisms.1,6,8,13 Major transient risk factors include surgeries requiring general anesthesia lasting ≥ 30 minutes, prolonged bed confinement in a hospital due to acute illness for three or more days, cesarean delivery, and major trauma.1,6,8,13 Minor transient factors include shorter surgeries, hospitalization for less than three days with an acute illness, estrogen therapy (oral contraceptives, hormone replacement), pregnancy, leg injuries restricting mobility for three or more days, and prolonged travel.1,6,8,13 Additionally, VTE risk is increased by factors such as smoking, advanced age (notably ≥ 40 years), and comorbidities like inflammatory bowel disease, antiphospholipid syndrome, active cancers (e.g., non-small cell lung, pancreatic, liver), and spinal cord injury.1,6,8,13 Understanding these factors is vital for clinical management and prevention of VTE, particularly in high-risk hospitalized patients.
Table 1. Risk Factors for Venous Thromboembolism1,6,8,13 | |
Mechanism | Risk Factor |
Venous Stasis |
|
Endothelial Injury |
|
Hypercoagulability |
|
BMI: body mass index | |
Risk Stratification Tools
Validated clinical tools are available for use in the diagnosis of VTE based on clinical features, namely Wells Criteria for DVT (see the criteria here: https://www.guidelinecentral.com/calculators/2c9e8038734e3c9e01735344777f005a/ and interpretation in Table 2), Wells Criteria for PE (see the criteria here: https://www.guidelinecentral.com/calculators/2c9e8038734e3c9e01735344770f0059/), and the Pulmonary Embolism Rule Out Criteria (PERC) (see the criteria here: https://www.mdcalc.com/calc/347/perc-rule-pulmonary-embolism). These tools use aspects of the history and physical exam for risk stratification and management of patients suspected of having VTE.14,15 Clinicians should use Wells Criteria to estimate the patient’s pretest probability of DVT and PE, which can guide subsequent testing and treatment. Table 3 highlights the differences between Wells Criteria for DVT and Wells Criteria for PE.
Table 2. Interpretation of Wells Criteria for Deep Vein Thrombosis19,20 | ||
Wells Score | Risk Group | Prevalence |
≤ 0 | Low/unlikely | 5% |
1-2 | Moderate | 17% |
≥ 2 | High/likely | 17% to 53% |
Table 3. Differences Between Wells Score for Deep Vein Thrombosis and Pulmonary Embolism | ||
Wells Score for DVT | Wells Score for PE | |
Focus | Limb symptoms + history | Respiratory symptoms + history |
Clinical Features | Swelling, pain | Tachycardia, shortness of breath, hemoptysis |
Imaging | Venous Doppler ultrasound | Chest CTA |
DVT: deep vein thrombosis; PE: pulmonary embolism; CTA: computed tomography angiography | ||
Wells Criteria for PE can be interpreted using a three-tier or two-tier model for risk stratification, with the two-tier model being recommended for clinical use.16 The PERC rule is meant for use in patients who already have been determined to be low-risk, where PE can be safely ruled out based on history and physical exam without further workup.17,18 Table 4 highlights the differences between Wells Criteria for PE and the PERC Rule.
Table 4. Differences Between Wells Score for Pulmonary Embolism and PERC Rule | ||
Wells Score for PE | PERC Rule | |
Use | Any suspicion of PE | Low suspicion of PE |
Purpose | Estimating pretest probability of PE | Ruling out PE in low-risk patients |
PE: pulmonary embolism; PERC: Pulmonary Embolism Rule Out Criteria | ||
Diagnostic Studies
When DVT or PE is suspected, the first step to obtaining diagnostic testing is to determine the pretest probability. There are multiple clinical prediction tools, such as Wells Criteria for DVT and Wells Criteria for PE listed earlier, that have been well-validated to determine this pretest probability.
Laboratory Testing
For patients with a low pretest probability of VTE, a D-dimer assay may be used. D-dimer is highly sensitive but has poor specificity.21 Other causes of elevated D-dimer include cancer, cardiovascular diseases, diabetes, infections, inflammation, liver disease, renal disease, trauma, and surgery. Additionally, aging, pregnancy, and intense physical exercise can lead to elevations.22
Conventionally, 500 mcg/L fibrinogen equivalent units (FEU) is used as the cutoff; however, an age-adjusted cutoff has been validated for safe use to exclude both DVT and PE in patients with low likelihood. The age-adjusted cutoff is calculated by multiplying the patient’s age by 10 for patients older than the age of 50 years.23–26 A negative D-dimer and low pretest probability exclude the diagnosis of VTE.21 For patients with either a high pretest probability or a positive D-dimer, imaging is indicated.
Imaging
Ultrasound is the most common modality used in the ED to evaluate for DVT. Whole leg ultrasonography is performed most frequently and has a higher negative predictive value compared to proximal vein ultrasonography, but it also can detect distal thrombi for which no treatment typically is indicated.27 Not all EDs have 24-hour access to consultative ultrasonography and point-of-care ultrasound can be performed in these cases to evaluate for DVT. The two-point technique tests for compressibility of the common femoral vein and the popliteal vein, while the three-point technique also includes the superficial femoral vein.28 (See Figure 1.) A meta-analysis showed similar sensitivity, specificity, and false-negative rates between the two techniques.29
Figure 1. Femoral Vein Thrombus/Deep Vein Thrombosis |
Point-of-care ultrasound image demonstrating a femoral vein (FV) thrombus/deep vein thrombosis (white arrow) |
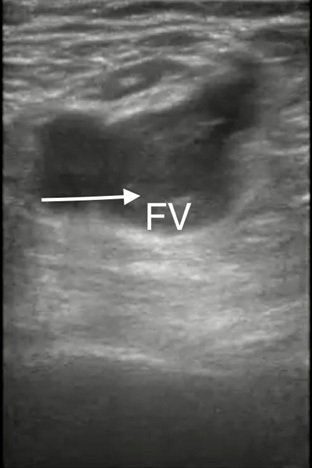 |
Image courtesy of Daniel Migliaccio, MD. |
For patients at high risk for PE or low-risk with a positive D-dimer, computed tomography angiography (CTA) is indicated. Contrast is used to assess for patency of the pulmonary arteries. Sensitivity of CTA for acute PE is 83%, with a specificity of 96% based on the PIOPED II trial.30 Advantages of CTA include that it is minimally invasive, widely available, and quick.31
Ventilation-perfusion (V/Q) scanning, which is a nuclear test, may be used in patients who have contraindications to CT imaging. V/Q scan interpretation depends on the criteria used. The modified PIOPED II criteria classify scans as PE present (high probability), nondiagnostic (low or intermediate probability), and PE absent (normal or very low probability) with sensitivity of 84.9% for “PE present” and specificity of 92.7% for “PE absent.”32 Alternatively, the PISAPED criteria classify scans into PE present or PE absent, with sensitivity of “PE present” 80.4% and specificity of “PE absent” 96.6%.32 Historically, V/Q has been used in pregnant patients; however, some local protocols favor CT in these patients because the radiation dose to the fetus is similar with more advanced CT machines.33
Echocardiography cannot be reliably used to diagnose PE; however, it can be used to assess for right ventricular (RV) dysfunction, which can be indicative of right heart strain from PE in patients with high suspicion.34 Point-of-care ultrasound frequently is used in the ED to evaluate for RV strain. Features such as the abnormal septal motion (D-sign), right ventricular hypokinesis (McConnell’s sign), and increased RV:LV ratio all have very low sensitivity but high specificity for PE in patients with concern for VTE.35 Additionally, a free-floating thrombus may be visualized in the right atrium or ventricle known as a clot-in-transit, which carries a high mortality.36 Evidence of right heart dysfunction or visualized thrombus can be used to guide empiric treatment in patients with high likelihood of PE who are too unstable to undergo additional imaging. (See Figures 2 and 3.)
Figure 2. Right Ventricle Dilation Secondary to Increased Right-Sided Heart Pressures |
Point-of-care ultrasound image demonstrating an apical four chamber view with RV dilation (white arrow) secondary to increased right-sided heart pressures |
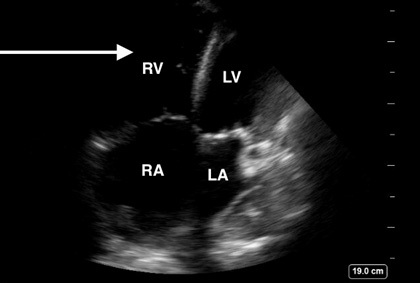 |
Image courtesy of Daniel Migliaccio, MD. |
Figure 3. Septal Bowing Caused by Right Ventricular Strain Demonstrating Classic D Sign |
Point-of-care ultrasound image demonstrating a parasternal short view with septal bowing (red arrow) caused by right ventricular strain demonstrating classic “D sign” |
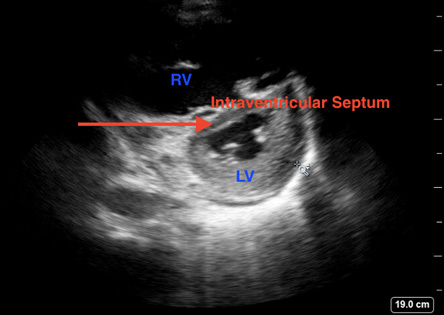 |
Image courtesy of Daniel Migliaccio, MD. |
Differential Diagnosis
The differential diagnosis for VTE can be broad-ranging from emergent, life-threatening pathology to VTE mimics. Table 5 has a list of differentials based on acuity for PE and DVT.
Table 5. Differential Diagnosis for Pulmonary Embolism and Deep Vein Thrombosis | |
Differential Diagnosis for Pulmonary Embolism | Life-Threatening/Emergent
Serious/Less Acute
Mimickers/Non-Acute/Chronic
|
Differential Diagnosis for Deep Vein Thrombosis | Life-Threatening/Emergent
Serious/Less Acute
Mimickers/Non-Acute/Chronic
|
MI: myocardial infarction; COPD: chronic obstructive pulmonary disease | |
Management of Venous Thromboembolism
Initial Management
Initial management considerations for DVT and PE depends on risk stratification and hemodynamic stability of the patient including ensuring patent airway, adequate breathing, and perfusing circulation. Anticoagulation remains the cornerstone of treatment for DVT and PE in patients who do not have a high bleeding risk.1 The American Heart Association (AHA) uses the terms “high-risk massive PE” (high risk), “high-risk submassive PE” (intermediate high risk), “low risk submassive PE” (intermediate low risk), and “low risk” PE. Hemodynamic instability (HDS) is defined as systolic blood pressure < 90 mmHg for > 15 minutes or cardiac arrest. HDS in a patient who has a confirmed PE diagnosis is sufficient to categorize a patient as high risk. Patients who fall into the low-risk category (see Table 6) can be discharged safely on anticoagulation after a risk-benefit discussion has occurred with the patient.37 Patients who fall into the high risk and intermediate-high categories may need other interventions to prevent further decompensation. RV strain or dysfunction as well as intracardiac clot can be visualized on cardiac ultrasound and CT pulmonary angiography (CTPA). Cardiac biomarkers such as N-terminal pro-B-type natriuretic peptide (NT-proBNP) typically are elevated (> 1,000) in the intermediate-high risk and high risk groups.38
Table 6. Risk Stratification of Early Mortality for Patients Presenting with Pulmonary Embolism | ||||
Mortality Risk | Hemodynamically Unstable? | Evidence of RV Strain or Intracardiac Thrombus? | sPESI ≥ 1? | |
Low | No | No | No | |
Intermediate | Intermediate low | No | No | Yes |
Intermediate high | No | Yes | Yes | |
High | Yes | Yes | Yes | |
RV: right ventricle; sPESI: simplified Pulmonary Embolism Severity Index | ||||
Hemodynamically Unstable
Similar to the management of other critically ill patients in the ED, the ABCDE (airway, breathing, circulation, disability, exposure) algorithm is a useful tool to ensure the most urgent patient needs are being addressed first.38 Patients with VTE who initially are hypoxic are given supplemental oxygen either via low-flow or high-flow mask depending on the patient’s needs to maintain oxygen levels ≥ 90%.39 Mechanical ventilation typically is regarded as a last resort for patients with worsening hypoxemia or impending cardiac or respiratory arrest. Induction agents can reduce systemic vascular resistance, further exacerbating hypotension in an already unstable patient. Positive pressure ventilation also increases intrathoracic pressure, reducing RV preload and potentially worsening RV failure.39
Fluid boluses are given in smaller aliquots (250 mL to 500 mL) to improve the cardiac index in hypotensive patients.39 Larger boluses can lead to RV overload and worsen right heart failure.39 Patients who are persistently hypotensive should be started on vasopressor therapy, typically with norepinephrine as the first-line agent.39
Regarding circulatory dysfunction due to obstructive shock, interventions available for the high-risk patient include systemic thrombolysis, catheter-directed therapy (suction embolectomy and catheter-directed thrombolysis), and surgical thrombectomy. Anticoagulation should be started in all high-risk patients as the discussion for thrombolysis is ongoing. The preferred agent for the high-risk patient is intravenous unfractionated heparin (UFH).8 The care team should weigh the risk of massive life-threatening bleeding from systemic thrombolysis against the risk of death from PE while taking into consideration the patient’s wishes. When available, PE response teams (PERTs) should be consulted to help guide management for high-risk and intermediate high-risk patients. PERTs typically include emergency medicine clinicians, intensivists, pulmonologists, cardiologists, interventional radiologists, thoracic and vascular surgeons, hematologists and pharmacists.40
In general, the diagnosis of PE should be confirmed with imaging before systemic thrombolysis is administered in the high-risk patient. For emergency cases where imaging is unable to be obtained because of the patient’s hemodynamic instability or cardiac arrest with ongoing cardiopulmonary resuscitation, systemic thrombolysis can be administered if there is a high index of suspicion for PE after bedside cardiac ultrasound has been performed.39 Intermediate high-risk patients with severe RV dysfunction due to PE, extensive clot burden, worsening hypoxemia or down-trending blood pressure, and rising heart rates also may be considered for thrombolytic therapy (systemic or catheter-directed) after weighing the risk of bleeding with the benefit of thrombolysis.39 Catheter-directed thrombolysis uses a low dose of thrombolytic therapy and theoretically carries a lower risk of major bleeding.41
Treatment Approach to Deep Vein Thrombosis
Anticoagulation is recommended for patients with acute proximal DVT (iliac, femoral, or popliteal vein) and acute symptomatic PE without a high risk of bleeding.1 This recommendation is based on studies that demonstrated more than 90% of acute PE cases arise from proximal veins.42 The approach for distal DVT management is less straightforward and depends on other patient risk factors since evidence for anticoagulation in this population is not as robust.8 Patients with acute distal DVT deemed at high risk for embolization should be offered anticoagulation.8 Table 7 enumerates factors that place patients with acute distal DVT at high risk of embolization and those who can undergo surveillance with serial compression ultrasound (CUS). CUS is done weekly for two weeks to assess for extension into the proximal veins.8 Patients with massive proximal DVT with high risk for early limb ischemia or limb loss (such as those with evidence of phlegmasia cerulea dolens) should be offered catheter-directed thrombolysis or surgical thrombectomy if there are contraindications to thrombolysis.43 Anticoagulation then is initiated in these patients after a period of observation in the inpatient setting.43 Risk factors for major bleeding, found in Table 8, must be considered prior to starting anticoagulation. The presence of two or more of these risk factors corresponds to a high risk (> 2% to 3% annually) of major bleeding.44
Table 7. Risk Stratification for Distal Deep Vein Thrombosis to Guide the Decision Between Anticoagulation and Surveillance with Compression Ultrasound40 | |
High-Risk Distal DVT | Low-Risk Distal DVT |
Symptomatic: leg pain, swelling, or discoloration | Asymptomatic |
Thrombus within 2 cm to the proximal popliteal vein | Non-diagnostic ultrasound |
Thrombus involving multiple distal veins | D-dimer < 500 ng/mL |
Unprovoked DVT | Thrombus in muscular veins |
Active cancer or persistent immobility | |
Personal history of DVT or PE | |
D-dimer ≥ 500 ng/mL | |
DVT: deep vein thrombosis; PE: pulmonary embolism | |
Table 8. Risk Factors for Major Bleeding While on Anticoagulation49 |
Risk factors for major bleeding while on anticoagulation
|
NSAIDs: nonsteroidal anti-inflammatory drugs |
Treatment Approach to Subsegmental Pulmonary Embolism
The rising availability and access to CT imaging has increased the number of patients diagnosed with subsegmental PE (SSPE).45 There is no consensus on whether patients with SSPE should receive anticoagulation because data are limited and practice differs among providers.46 A retrospective study showed that adverse events were similar between those with more proximal emboli and patients with SSPE.47 A prospective study looking at patients with isolated SSPE demonstrated a recurrent PE risk of 3.1% at 90 days, which is higher than the approximately 1% risk in the general population.48 In light of the findings from limited data on SSPE, anticoagulation is recommended in this category, especially if PE is unprovoked, the patient is symptomatic, there are persistent risk factors (e.g., malignancy), or significant cardiopulmonary comorbidities are present.8
Risk Stratification for Pulmonary Embolism
Select patients in the low-risk category (see Table 6) who have demonstrated stability after a period of observation in the hospital while being anticoagulated can be treated in the outpatient setting.37 The Simplified Pulmonary Severity Index Score (sPESI) and HESTIA criteria, seen in Table 9, are helpful clinical decision tools to identify risk of death within 30 days and patients who can safely undergo outpatient anticoagulation therapy for PE and DVT, respectively.53,54 An sPESI score of 0 indicates a low risk of mortality in 30 days, while any value > 1 indicates that the patient is high risk.1,49 Both the sPESI and Hestia criteria performed similarly in risk stratification and prognostic accuracy for 30-day mortality after PE, although the objective sPESI had a higher interobserver reliability. The American Board of Emergency Medicine recommends outpatient management of PE for patients who have no high or intermediate risk features and are low-risk by Hestia criteria or have an sPESI score of 0 combined with physician judgment (favorable overall medical and social situation for home treatment). High-risk features include signs of shock, end-organ damage, and hypotension, while intermediate-risk includes evidence of right heart strain, elevated troponin, or elevated BNP.51
Table 9. sPESI Score and Hestia Criteria49,50 | |
Simplified Pulmonary Severity Index (sPESI) Score | HESTIA Criteria |
Age > 80 years | Hemodynamic instability (systolic blood pressure < 100 mmHg) |
History of cancer | Need for thrombolysis or embolectomy |
Chronic cardiopulmonary disease | Active bleeding or high risk of bleeding |
Heart rate ≥ 110 beats per minute | Severe pain requiring intravenous analgesia |
Systolic blood pressure < 100 mmHg | Severe renal impairment with creatinine clearance < 30 mL/min |
Arterial oxygen < 90% | Severe liver impairment |
Platelet count < 100,000 mm³ | |
Recent trauma or surgery within two weeks | |
History of heparin-induced thrombocytopenia (HIT) | |
Pregnancy | |
Other medical/social reason requiring hospitalization (e.g., lack of support at home, inability to follow up) | |
In general, patients without high risk of bleeding on anticoagulation (see Table 8), severe cardiopulmonary comorbidities, severe renal insufficiency, need for supplemental oxygen or narcotics for pain control, and with adequate access to anticoagulation and outpatient monitoring can be offered outpatient therapy.49 Patients with massive DVTs (iliofemoral DVT) with or without concurrent PE, and concern for limb ischemia (e.g., phlegmasia cerulea dolens) are not appropriate for outpatient therapy.49 Clinical judgment in combination with patient preference should be considered when deciding on whether to initiate outpatient therapy for patients diagnosed with acute VTE.
Approach to Outpatient Management of Venous Thromboembolism
Evidence for outpatient anticoagulation is derived from meta-analyses and prospective studies that demonstrated similar rates of mortality, VTE , and major bleeding in patients treated outpatient vs. inpatient.4,52,53 The advent of oral DOACs has increased the convenience and feasibility of outpatient therapy because of their lack of need for frequent laboratory monitoring, quick onset of action, and lower bleeding risk when compared with warfarin.8 However, recent studies have demonstrated that outpatient therapy may be underused for low-risk patients in whom outpatient anticoagulation is appropriate.54-56 One cross-sectional study showed no significant change in discharge rates between 2012 (38%) and 2020 (33%).56 Of the patients who were considered low-risk, only one-third were discharged home on outpatient therapy. Other factors that may have limited outpatient therapy include patient comorbidities, inability to afford or access medication, and inadequate social support.56 Vinson et al performed a controlled pragmatic trial to test the utility of an integrated electronic clinical decision tool for increasing the rate of outpatient anticoagulation.55 The study found a statistically significant increase in the rate of outpatient therapy (17.4% vs. 28%).55 The results from this trial are promising, but more data are needed to elucidate the true impact of clinical decision tools integrated into the electronic medical record (EMR) on the rate of outpatient therapy.
Patients in the intermediate low-risk category typically require admission for a period of observation while on anticoagulation prior to discharge. Low-risk patients can be discharged safely on anticoagulation after discussing the risks and benefits of anticoagulation with the patient while incorporating their preferences into the plan of care.1 Factors that determine the agent for anticoagulation in low-risk patients include the presence of active cancer, pregnancy, evidence of thrombophilia, renal function, liver function, compliance, cost, and history of gastrointestinal bleeding.8
Anticoagulation
Anticoagulation includes an initiation phase, treatment or long-term phase, and occasionally an extended phase.8 Table 10 is a list of treatment options with clinical considerations. Options for the initiation phase include low molecular weight (LMW) heparin, fondaparinux, UFH, and direct factor Xa inhibitors.8 The preferred agent used in the treatment phase determines which agent is selected for the initiation phase.
Table 10. Outpatient Treatment Options for Venous Thromboembolism8,13,35,52 | |
Treatment Option | Clinical Considerations |
Direct oral anticoagulants (DOACs) |
|
Low molecular weight heparin (LMWH) |
|
Vitamin K antagonists (e.g., warfarin) |
|
Clinical curveillance |
|
There is an initiation phase and a treatment phase for the majority of anticoagulation medications, as noted in Table 11. Oral monotherapy with a direct factor Xa inhibitor, such as apixaban or rivaroxaban, is the most common strategy for anticoagulation in low-risk patients with intact renal function (creatinine clearance [CrCl] ≥ 30 mL/minute) and gastrointestinal absorption, but other strategies exist, such as sequential therapy, overlapping therapy, and parenteral monotherapy.8 Apixaban oral monotherapy in the initiation phase typically is dosed as 10 mg twice daily for seven days, while rivaroxaban in the initiation phase is 15 mg twice daily for 21 days.8 Sequential therapy begins with LMW heparin for at least five days in the initiation phase before switching to the dabigatran or edoxaban in the treatment phase. LMW heparin dosing varies by the subtype (enoxaparin, dalteparin, tinzaparin). Enoxaparin can be dosed twice daily 1 mg/kg or once daily 1.5 mg/kg.57
Table 11. Dosing of Anticoagulation for Venous Thromboembolism8,13,35,52 | |||
Medication | Initial Phase | Short-Term Phase (3-6 months) | Indefinite Phase (after 3-6 months) |
Apixaban | 10 mg twice a day for 7 days | 5 mg twice a day | 5 mg twice a day or 2.5 mg twice a day |
Rivaroxaban | 15 mg twice a day for 21 days | 20 mg once a day | 20 mg once a day or 10 mg once a day |
Dabigatran | Administered after 5-10 days of parenteral anticoagulation | 150 mg twice a day | |
Edoxaban | Administered after 5-10 days of parenteral anticoagulation | 60 mg once a day | |
LMWH | 1 mg/kg twice a day or 1.5mg/kg once daily Administered subcutaneously for a minimum of 5 days | ||
Warfarin | Administered concurrently with LMWH until INR ≥ 2 for 2 days | Target INR of 2 to 3 | |
LMWH: low molecular weight heparin; INR: international normalized ratio | |||
Overlapping therapy is used when the desired treatment agent is warfarin. Warfarin therapy is begun on the same day following a parenteral agent. The parenteral agent is continued until the international normalized ratio (INR) has been ≥ 2.0 for at least 24 hours.8 Parenteral monotherapy is selected for patients who are unable to tolerate oral therapy or require parenteral therapy, such as in select patients with cancer.8 For most patients in the low-risk category with otherwise intact renal function (CrCl ≥ 30 mL/minute) without high bleeding risk, monotherapy with direct factor Xa inhibitors may be the most optimal treatment option. Recent studies have shown that factor Xa inhibitors (specifically apixaban, rivaroxaban, and edoxaban) have similar efficacy and bleeding risk when compared to conventional overlapping therapy with LMW heparin and warfarin.58-60
The AMPLIFY trial was a prospective, randomized, double-blind study that compared apixaban (initiation phase dose for seven days followed by treatment phase dose of 5 mg twice daily for six months) with conventional overlapping therapy (warfarin plus subcutaneous LMW heparin bridge therapy) in the acute treatment of VTE for 5,395 patients.60 Apixaban therapy in this trial was shown to be noninferior to warfarin plus subcutaneous bridge therapy and had significant reduction in the risk of major bleeding.60 A larger scale matched-cohort analysis (35,750 patients) focusing on acute VTE treatment in the outpatient setting also compared apixaban therapy with warfarin plus subcutaneous LMW heparin bridge therapy. The study showed significant reduction in the risk of recurrent VTE and major bleeding for the apixaban group.59 Oral factor Xa inhibitors are attractive agents in the initiation phase because of their ease of use for patients, quick onset of action (peak therapeutic levels within one to four hours of ingestion), lack of need for frequent laboratory monitoring, and lower risk of bleeding when compared with warfarin, albeit more costly.8
Clinical Considerations when Choosing Direct Oral Anticoagulants
While DOACs are first-line agents for most patients because of their ease of use, safety and efficacy, and without the required laboratory monitoring, there are certain situations when they are not recommended. Renal impairment, hepatic impairment, and extremes of body weight may alter DOAC pharmacodynamics.61 Apixaban failed to demonstrate noninferiority to warfarin in the PROACT Xa trial for patients with mechanical heart valves, and warfarin remains the recommended agent in these patients.62 Additionally, drug to drug interactions may impede the use of DOACs.61 Table 12 is a curated list of contraindications.
Table 12. Contraindications to Direct Oral Anticoagulants56,58 | |
Category | Contraindication |
Renal impairment |
|
Hepatic impairment |
|
Specific conditions |
|
Drug interactions |
|
Gastrointestinal conditions |
|
Body weight extremes |
|
Other special populations |
|
Duration of Anticoagulation
The goal of anticoagulation in patients with VTE is to prevent embolization, recurrent thrombosis, and death, with the greatest risk occurring within the first three to six months after diagnosis.13 Treatment phase of anticoagulation usually lasts three to six months after the initiation phase.13 Options for the treatment phase include factor Xa inhibitors (e.g., apixaban, rivaroxaban, edoxaban), LMW heparin, fondaparinux, warfarin, and direct thrombin inhibitors (e.g., dabigatran).39 Coadministration with LMW heparin is not required for apixaban and rivaroxaban in the initiation phase as opposed to edoxaban and dabigatran, which require at least five days of LMW heparin. Edoxaban and dabigatran therapy can be started immediately after UFH infusion is stopped or when the next LMW heparin would have been due.13
Dosing of medication is variable depending on if the patient is being initiated, undergoing short-term vs. indefinite treatment (see Table 11). For treatment phase anticoagulation, apixaban typically is dosed 5 mg twice daily, rivaroxaban is dosed at 20 mg daily, edoxaban is dosed at 60 mg daily, and dabigatran is dosed at 150 mg daily.13 Unlike most DOACs, including factor Xa inhibitors and direct thrombin inhibitors, that are contraindicated in advanced renal failure (CrCl < 30L/minute), apixaban is approved for therapy in all stages of kidney disease.63 In patients with severe renal insufficiency (CrCl < 30 L/minute), an initiation phase with intravenous UFH for four to six days and then transitioning to apixaban if there is no high risk of major bleeding is recommended.63
Most patients with acute VTE will need anticoagulation for at least three months.8 The treatment phase may be longer in certain populations, such as pregnant patients with acute VTE, patients with active cancer, and patients with thrombus at unusual locations (e.g., cerebral or splanchnic veins).13 At the conclusion of the selected duration of the treatment phase, a decision is made whether patients are offered extended phase (indefinite) therapy for secondary prevention of recurrent VTE.13 This decision takes into account whether VTE was provoked, the patient’s comorbidities, and bleeding risk. Clinical decision tools like the HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly) score and HERDOO2 (hyperpigmentation, edema or redness in either leg; D-dimer level ≥ 250 mcg/L; obesity with body mass index ≥ 30, or older age) have been shown to be helpful in determining the need for further anticoagulation depending on competing risks of bleeding and recurrent VTE, respectively.64,65 The RIETE (registro informatizado de la enfermedad tromboembolica) and VTE-BLEED (venous thromboembolism bleeding) scores also are validated clinician decision tools for estimating bleeding risk in patients with acute VTE started on anticoagulation.66,67 The recommendation for a minimum anticoagulation period of three months is based on randomized controlled trials and meta-analyses mostly involving patients with both provoked and unprovoked proximal DVTs and PE. These studies showed that the risk of recurrent VTE is highest within the first three months and that reducing the length of anticoagulation to four to six weeks was associated with increased risk of VTE even in patients with distal DVTs.68-71 There is not as much data or strong evidence for patients with isolated distal DVTs, or asymptomatic or incidental subsegmental PEs, especially in cases that are deemed provoked. Nevertheless, based on the data from proximal DVTs and symptomatic PEs, anticoagulation is recommended for at least three months.68-71
Venous Thromboembolism Treatment in Special Populations
The second leading cause of death in patients with malignancy, after death from the malignancy itself, is VTE.72 Thus, patients with active cancer are at high risk of developing VTE and should be considered for anticoagulation therapy if there is no high risk of bleeding. The International Society of Thrombosis and Hemostasis defines active cancer as cancer diagnosed within the last six months, metastatic or regionally advanced, hematologic cancer not in complete remission, or cancer for which treatment has been given within the last six months.73 Patients with cancer who have been diagnosed with DVT (without PE) can be treated on an outpatient basis if they are ambulatory, have a low bleeding risk, do not have severe renal insufficiency, and have adequate access to medication and outpatient follow-up.73 Those who are diagnosed with PE that is thought to be provoked by their underlying malignancy usually require an inpatient stay for observation even if considered low risk prior to discharge home on outpatient anticoagulation.73
LMW heparin used to be the first-line agent for anticoagulation in patients with malignancy until the advent of DOACs.73 Oral factor Xa inhibitors (apixaban, rivaroxaban, edoxaban), in particular, have been shown to be similarly efficacious when compared with LMW heparin in patients with cancer.74-77 The CARAVAGGIO trial was a randomized controlled trial that compared apixaban monotherapy (initiation phase for seven days and treatment phase for six months) with subcutaneous dalteparin (200 units/kg for one month and then 150 units/kg daily) in 1,170 patients with active cancer and VTE.74 The study showed no statistically significant difference in the rate of recurrent VTE or major bleeding between apixaban monotherapy or LMW heparin monotherapy.74 Some studies have demonstrated lower bleeding risk with apixaban when compared with other DOACs and LMW heparin in patients with gastrointestinal malignancy, but the evidence for this is not clear since other studies have demonstrated conflicting results.74,78-80 Patients with cancer-provoked VTE who also have severe renal insufficiency (CrCl < 30 L/minute) with contraindications to renally dosed anticoagulants are started on overlapping therapy with intravenous UFH and warfarin.81
Regarding duration of therapy, most patients with cancer-provoked VTE will need anticoagulation for at least three to six months.81 Those who present with symptomatic PE or symptomatic proximal DVT thought to have been provoked by cancer will need anticoagulation indefinitely.81 Patients with cancer with other transient provoking factors (such as surgery) or who develop a major bleeding complication should not be on extended phase anticoagulation.81 Patients who also have been identified to be in durable remission also can have their anticoagulation discontinued.81
Pregnancy and the postpartum period are associated with increased risk of VTE.82 The indications for starting anticoagulation in pregnant and postpartum patients are similar to indications for the general population. Pregnant patients with a proximal DVT or PE without limb ischemia, massive DVT (e.g., phlegmasia cerulea dolens), or high bleeding risk should be started on anticoagulation.82 The evidence for the need for anticoagulation in pregnant patients with distal DVTs or SSPE is less certain; however, anticoagulation is recommended in these patients because of the inherent risk of VTE in the pregnant and postpartum period.82 Similar to the general population, pregnant patients are deemed low-risk (bleeding risk < 2%) or high risk (bleeding risk > 13%) based on their comorbidities and risk factors, such as platelet count, presence of renal disease, presence of liver disease, and fall risk (see Table 8).8 In general, the agent of choice for anticoagulation in pregnant patients is subcutaneous LMW heparin because warfarin and DOACs are contraindicated in pregnancy due to their potential risk of harm to the fetus.82 It is important to incorporate a multidisciplinary team approach, including maternal-fetal medicine, obstetric anesthesia, hematology, neonatology, and the PE response team in the management of pregnant and postpartum patients with diagnosed VTE. In the postpartum period in lactating mothers, LMW heparin remains the first choice because DOACs generally are avoided during breastfeeding.83 For patients who are not lactating in the postpartum period, DOACs are preferred because of their ease of use.83
Management of VTE in patients with prothrombotic disorders is complex and depends on the individual patient’s comorbidities and the presence of other provoking factors. Antiphospholipid syndrome and antithrombin deficiency are considered major prothrombotic conditions and, thus, require indefinite anticoagulation.84,85 Patients who are heterozygous for the Factor V Leiden mutation or prothrombin gene mutation do not require indefinite anticoagulation because these disorders are considered lower risk thrombophilias.86 There is no strong evidence to recommend indefinite anticoagulation in patients with protein C or S deficiency.86 Extended phase overlapping therapy with LMW heparin and warfarin is the recommended first-line agent for patients with diagnosed antiphospholipid syndrome and hereditary antithrombin deficiency.84,85 For the general population, individuals who develop heparin-induced thrombocytopenia while on LMW heparin outpatient should be switched to a DOAC.87
Inferior Vena Cava Filters
Patients with acute lower extremity DVT or PE with contraindications to anticoagulation or high bleeding risk should be considered for inferior vena cava (IVC) filters.88 For patients with acute PE in this category, IVC filters still are recommended even in the absence of diagnosed lower extremity DVT since thrombus can remain undetected in the calf veins or pelvis and can reform rapidly in the lower extremity after initial embolization.88 IVC filters also are recommended for patients on anticoagulation with recurrent DVTs, patients with large emboli burden in the pulmonary arteries in addition to proximal DVT, and those in whom another embolic event would be catastrophic.88
Retrievable IVC filters are recommended over permanent filters because IVC filters themselves are associated with complications such as thrombosis at the filter insertion site, filter fracture, embolization of filter fragments, hemorrhage, and guidewire entrapment.89 The overall rate of IVC filter placement has decreased, and the rate of filter retrieval is rising steadily.90 IVC filters should be retrieved once there is no longer a contraindication to anticoagulation or when there is no longer an indication for an IVC filter as deemed by the outpatient care team.89 The recommendations for IVC filters are based on studies that showed a low short-term PE recurrence rate in patients with acute DVT.88,91,92 Data for the efficacy of IVC filter in acute PE, on the other hand, are limited and do not show strong evidence for long-term survival benefit in patients with acute PE.91,93,94
Elastic/Graduated Compression Stockings
Graduated compression stockings can be used to treat the symptoms of post-thrombotic syndrome (PTS), a chronic condition that occurs when venous valves are destroyed by DVT, leading to edema, pain, and varicose veins.1 Smaller trials have reported up to 50% reduction in the risk of PTS when graduated compression stockings are used prophylactically after acute DVT diagnosis, but one large randomized trial showed no benefit when graduated compression stockings were worn by patients over a two-year span.95,96 Hence, routine use of graduated compression stockings in patients with acute DVT for the prevention of PTS is not indicated. When graduated compression stockings are used for symptom control, they should be started only after anticoagulation has been initiated owing to the theoretical risk of embolization. The recommended duration for graduated compression stockings is two years with replacement every six months and refitting performed as needed as edema resolves.13 Early ambulation is encouraged in patients with acute DVT on anticoagulation with well-controlled pain to reduce the risk of PTS.97
Outpatient Monitoring
Patients are monitored in the outpatient setting to ensure that their initial presenting symptoms are improving, that they are therapeutic on anticoagulation, to make sure they have not developed any contraindications to anticoagulation, and to assess for any complications such as VTE recurrence or major bleeding.8 DOACs and LMW heparins, as opposed to warfarin, do not require outpatient laboratory tests to ensure therapeutic levels. The INR is checked every three to four weeks for patients on warfarin therapy for a goal of 2 to 3.98 Conditions that could be contraindications or affect the half-life of anticoagulation, such as renal failure, pregnancy, or weight gain, are assessed to guide therapy.99 Patients also are monitored for adverse events like major bleeding (from anticoagulation), thrombocytopenia (from heparin), and skin necrosis (from warfarin).
Meta-analyses and randomized trials involving patients with VTE on DOACs showed a major bleeding risk ranging from 0.6% to 1.6%.100-102 These studies also showed a statistically lower risk of major bleeding events when DOACs were compared with overlapping therapy with heparin plus warfarin.100-102 Patients with suspected recurrent DVT or PE should undergo repeat CUS or CTPA, respectively.99 The most common reason for recurrent VTE is subtherapeutic anticoagulation, but other etiologies should be investigated, such as underlying malignancy, inherited thrombotic disorder (e.g., protein C deficiency), antiphospholipid syndrome, and May-Thurner syndrome.99 Subtherapeutic anticoagulation could be secondary to malabsorption, medication nonadherence, altered dose requirement due to weight gain or renal function, and incorrect medication dosage.99
Consultation with a coagulation specialist/hematologist is crucial in these cases to determine the optimal anticoagulation dose, agent for anticoagulation and for any required further testing.99 Those who experience persistent or worsening dyspnea may have developed chronic thromboembolic disease (CTED) or chronic thromboembolic pulmonary hypertension (CTEPH) and will need a referral to a CTEPH specialty clinic/center for definitive diagnosis and further management.103 Patients diagnosed with CTEPH remain on anticoagulation indefinitely if there is no high risk of major bleeding and may be managed medically or surgically with pulmonary artery thromboendarterectomy (PTE).103 PTE is considered the only definitive therapy for CTEPH, but it is only available to patients who are considered appropriate surgical candidates.103
Summary
VTE, which includes DVT and PE, is a common presentation in the ED. A systematic approach, incorporating the patient’s history, risk factors, physical examination findings, and validated risk stratification tools, can effectively guide diagnostic evaluation. Many patients with VTE can be safely managed in the outpatient setting. DOACs are the preferred treatment for most patients; however, clinicians must remain mindful of contraindications that may necessitate alternative therapies.
Jessica Wanthal, MD, is Assistant Professor, Department of Emergency Medicine, Duke University Medical Center, Durham, NC.
Megan Rivera, MD, is Assistant Professor, Department of Emergency Medicine, Duke University Medical Center, Durham, NC.
William Osae, MD, is Resident Physician, Duke University Medical Center, Department of Emergency Medicine, Durham, NC.
Sreeja Natesan, MD, is Associate Program Director, Department of Emergency Medicine, Duke University Medical Center, Durham, NC.
References
1. Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet. 2021;398(10294):64-77.
2. Freund Y, Cohen-Aubart F, Bloom B. Acute pulmonary embolism: A review. JAMA. 2022;328(13):1336-1345.
3. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Thromboembolic Disease; Wolf SJ, Hahn SA, Nentwich LM, et al. Clinical Policy: Critical Issues in the Evaluation and Management of Adult Patients Presenting to the Emergency Department With Suspected Acute Venous Thromboembolic Disease. Ann Emerg Med. 2018;71(5):e59-e109.
4. Yoo HH, Nunes-Nogueira VS, Fortes Villas Boas PJ, Broderick C. Outpatient versus inpatient treatment for acute pulmonary embolism. Cochrane Database Syst Rev. 2022;5(5):CD010019.
5. Expert Panel on Cardiac Imaging; Kirsch J, Wu CC, Bolen MA, et al. ACR Appropriateness Criteria® Suspected Pulmonary Embolism: 2022 Update. J Am Coll Radiol. 2022;19(11S):S488-S501.
6. Pastori D, Cormaci VM, Marucci S, et al. A comprehensive review of risk factors for venous thromboembolism: From epidemiology to pathophysiology. Int J Mol Sci. 2023;24(4):3169.
7. Peacock WF, Singer AJ. Reducing the hospital burden associated with the treatment of pulmonary embolism. J Thromb Haemost. 2019;17(5):720-736.
8. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: Second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160(6):e545-e608.
9. Maughan BC, Frueh L, McDonagh MS, et al. Outpatient treatment of low-risk pulmonary embolism in the era of direct oral anticoagulants: A systematic review. Acad Emerg Med. 2021;28(2):226-239.
10. Phillippe HM. Overview of venous thromboembolism. Am J Manag Care. 2017;23(20 Suppl):S376-S382.
11. Sternbach G. John Homans: The dorsiflexion sign. J Emerg Med. 1989;7(3):287-290.
12. Ambesh P, Obiagwu C, Shetty V. Homan’s sign for deep vein thrombosis: A grain of salt? Indian Heart J. 2017;69(3):418-419.
13. Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693-4738.
14. Wells PS, Hirsh J, Anderson DR, et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet. 1995;345(8961):1326-1330.
15. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350(9094):1795-1798.
16. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29(18):2276-2315.
17. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780.
18. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: Best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711.
19. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e351S-e418S.
20. Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295(2):199-207.
21. Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: An update. N Am J Med Sci. 2014;6(10):491-499.
22. Franchini M, Focosi D, Pezzo MP, Mannucci PM. How we manage a high D-dimer. Haematologica. 2024;109(4):1035-1045.
23. Nybo M, Hvas AM. Age-adjusted D-dimer cut-off in the diagnostic strategy for deep vein thrombosis: A systematic review. Scand J Clin Lab Invest. 2017;77(8):568-573.
24. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: The ADJUST-PE Study. JAMA. 2014;311(11):1117-1124.
25. Robert-Ebadi H, Robin P, Hugli O, et al. Impact of the age-adjusted D-dimer cutoff to exclude pulmonary embolism: A multinational prospective real-life study (the RELAX-PE study). Circulation. 2021;143(18):1828-1830.
26. Douma RA, Tan M, Schutgens REG, et al. Using an age-dependent D-dimer cut-off value increases the number of older patients in whom deep vein thrombosis can be safely excluded. Haematologica. 2012;97(10):1507-1513.
27. Stevens SM, Fazili M, Woller SC. Choosing ultrasound technique for suspected deep vein thrombosis: Which is best? Quant Imaging Med Surg. 2020;10(6):1418-1421.
28. Varrias D, Palaiodimos L, Balasubramanian P, et al. The use of point-of-care ultrasound (POCUS) in the diagnosis of deep vein thrombosis. J Clin Med. 2021;10(17):3903.
29. Lee JH, Lee SH, Yun SJ. Comparison of 2-point and 3-point point-of-care ultrasound techniques for deep vein thrombosis at the emergency department: A meta-analysis. Medicine (Baltimore). 2019;98(22):e15791.
30. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354(22):2317-2327.
31. Moore AJE, Wachsmann J, Chamarthy MR, et al. Imaging of acute pulmonary embolism: An update. Cardiovasc Diagn Ther. 2018;8(3):225-243.
32. Sostman HD, Miniati M, Gottschalk A, et al. Sensitivity and specificity of perfusion scintigraphy combined with chest radiography for acute pulmonary embolism in PIOPED II. J Nucl Med. 2008;49(11):1741-1748.
33. Tester J, Rees M, Pascoe D, et al. Diagnostic imaging for suspected pulmonary embolism during pregnancy and postpartum: A comparative radiation dose study. J Med Imaging Radiat Oncol. 2023;67(3):223-231.
34. Dabbouseh NM, Patel JJ, Bergl PA. Role of echocardiography in managing acute pulmonary embolism. Heart. 2019;105(23):1785-1792.
35. Fields JM, Davis J, Girson L, et al. Transthoracic echocardiography for diagnosing pulmonary embolism: A systematic review and meta-analysis. J Am Soc Echocardiogr. 2017;30(7):714-723.e4.
36. Igwilo R, Pinsino A, Aksan F, Kapoor S. Clot-in-transit: A ticking time bomb in the heart with serious consequences. SAGE Open Med Case Rep. 2023;11:2050313X231151504.
37. Khatib R, Nitti K, McDowell M, et al. Outpatient management of patients presenting with venous thromboembolism: Retrospective cohort study at 11 community hospitals. J Thromb Thrombolysis. 2021;52(1):179-188.
38. Thim T, Krarup NHV, Grove EL, et al. Initial assessment and treatment with the Airway, Breathing, Circulation, Disability, Exposure (ABCDE) approach. Int J Gen Med. 2012;5:117-121.
39. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543-603.
40. Fleitas Sosa D, Lehr AL, Zhao H, et al. Impact of pulmonary embolism response teams on acute pulmonary embolism: A systematic review and meta-analysis. Eur Respir Rev. 2022;31(165):220023.
41. Engelberger RP, Kucher N. Catheter-based reperfusion treatment of pulmonary embolism. Circulation. 2011;124(19):2139-2144.
42. Galanaud JP, Sevestre-Pietri MA, Bosson JL, et al. Comparative study on risk factors and early outcome of symptomatic distal versus proximal deep vein thrombosis: Results from the OPTIMEV study. Thromb Haemost. 2009;102(3):493-500.
43. Mazzolai L, Aboyans V, Ageno W, et al. Diagnosis and management of acute deep vein thrombosis: A joint consensus document from the European Society of Cardiology Working Groups of Aorta and Peripheral Vascular Diseases and Pulmonary Circulation and Right Ventricular Function. Eur Heart J. 2018;39(47):4208-4218.
44. Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135(5):317-325.
45. Bariteau A, Stewart LK, Emmett TW, Kline JA. Systematic review and meta-analysis of outcomes of patients with subsegmental pulmonary embolism with and without anticoagulation treatment. Acad Emerg Med. 2018;25(7):828-835.
46. Yoo HH, Nunes-Nogueira VS, Fortes Villas Boas PJ. Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database Syst Rev. 2020;2(2):CD010222.
47. Raslan IA, Chong J, Gallix B, et al. Rates of overtreatment and treatment-related adverse effects among patients with subsegmental pulmonary embolism. JAMA Intern Med. 2018;178(9):1272-1274.
48. Le Gal G, Kovacs MJ, Bertoletti L, et al. Risk for recurrent venous thromboembolism in patients with subsegmental pulmonary embolism managed without anticoagulation: A multicenter prospective cohort study. Ann Intern Med. 2022;175(1):29-35.
49. Aujesky D, Roy PM, Verschuren F, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: An international, open-label, randomised, non-inferiority trial. Lancet. 2011;378(9785):41-48.
50. Zondag W, Mos ICM, Creemers-Schild D, et al. Outpatient treatment in patients with acute pulmonary embolism: The Hestia Study. J Thromb Haemost. 2011;9(8):1500-1507.
51. Brown M, Wolf S, Yealy D. Key Advances Clinical Policy Alert: Outpatient Treatment for Pulmonary Embolism. American Board of Emergency Medicine. May 2024. https://www.abem.org/wp-content/uploads/2024/07/key-advances_pulmonary-embolism_clinical-policy-alert.pdf
52. Peacock FW, Coleman CI, Diercks DB, et al. Emergency department discharge of pulmonary embolus patients. Acad Emerg Med. 2018;25(9):995-1003.
53. Barco S, Schmidtmann I, Ageno W, et al. Early discharge and home treatment of patients with low-risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban: An international multicentre single-arm clinical trial. Eur Heart J. 2020;41(4):509-518.
54. Stein PD, Matta F, Hughes PG, et al. Home treatment of pulmonary embolism in the era of novel oral anticoagulants. Am J Med. 2016;129(9):974-977.
55. Vinson DR, Mark DG, Chettipally UK, et al. Increasing safe outpatient management of emergency department patients with pulmonary embolism: A controlled pragmatic trial. Ann Intern Med. 2018;169(12):855-865.
56. Watson NW, Carroll BJ, Krawisz A, et al. Trends in discharge rates for acute pulmonary embolism in U.S. emergency departments. Ann Intern Med. 2024;177(2):134-143.
57. Hacobian M, Shetty R, Niles CM, et al. Once daily enoxaparin for outpatient treatment of acute venous thromboembolism: A case-control study. Clin Appl Thromb Hemost. 2010;16(1):21-25.
58. Hokusai-VTE Investigators; Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406-1415.
59. Weycker D, Li X, Wygant GD, et al. Effectiveness and safety of apixaban versus warfarin as outpatient treatment of venous thromboembolism in U.S. clinical practice. Thromb Haemost. 2018;118(11):1951-1961.
60. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808.
61. Chen A, Stecker E, Warden B. Direct oral anticoagulant use: A practical guide to common clinical challenges. J Am Heart Assoc. 2020;9(13):e017559.
62. Mack CA, Lau C, Girardi LN. There is still no alternative to warfarin for mechanical valves: It remains the most effective anticoagulant. J Thorac Cardiovasc Surg. 2024; Jul 14:S0022-5223(24)00610-X. doi:10.1016/j.jtcvs.2024.07.011. [Online ahead of print].
63. Tham D, Zhao L, Yu W, et al. Safety and efficacy of direct oral anticoagulants in chronic kidney disease: A meta-analysis. Res Pract Thromb Haemost. 2024;8(7):102584.
64. Lip GYH, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: The HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57(2):173-180.
65. Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: Multinational prospective cohort management study. BMJ. 2017;356:j1065.
66. Klok FA, Hösel V, Clemens A, et al. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur Respir J. 2016;48(5):1369-1376.
67. Ruíz-Giménez N, Suárez C, González R, et al; RIETE Investigators. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100(1):26-31.
68. Baglin T, Bauer K, Douketis J, et al. Duration of anticoagulant therapy after a first episode of an unprovoked pulmonary embolus or deep vein thrombosis: Guidance from the SSC of the ISTH. J Thromb Haemost. 2012;10(4):698-702.
69. Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: Analysis of individual participants’ data from seven trials. BMJ. 2011;342:d3036.
70. Ageno W, Bertù L, Bucherini E, et al. Rivaroxaban treatment for six weeks versus three months in patients with symptomatic isolated distal deep vein thrombosis: Randomised controlled trial. BMJ. 2022;379:e072623.
71. Franco L, Giustozzi M, Agnelli G, Becattini C. Anticoagulation in patients with isolated distal deep vein thrombosis: A meta-analysis. J Thromb Haemost. 2017;15(6):1142-1154.
72. Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632-634.
73. Khorana AA, Noble S, Lee AYY, et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(9):1891-1894.
74. Agnelli G, Becattini C, Meyer G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599-1607.
75. McBane RD 2nd, Wysokinski WE, Le-Rademacher JG, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J Thromb Haemost. 2020;18(2):411-421.
76. Planquette B, Bertoletti L, Charles-Nelson A, et al. Rivaroxaban vs dalteparin in cancer-associated thromboembolism: A randomized trial. Chest. 2022;161(3):781-790.
77. Riaz IB, Fuentes HE, Naqvi SAA, et al. Direct oral anticoagulants compared with dalteparin for treatment of cancer-associated thrombosis: A living, interactive systematic review and network meta-analysis. Mayo Clin Proc. 2022;97(2):308-324.
78. Agnelli G, Muñoz A, Franco L, et al. Apixaban and dalteparin for the treatment of venous thromboembolism in patients with different sites of cancer. Thromb Haemost. 2022;122(5):796-807.
79. Ageno W, Vedovati MC, Cohen A, et al. Bleeding with apixaban and dalteparin in patients with cancer-associated venous thromboembolism: Results from the Caravaggio Study. Thromb Haemost. 2021;121(5):616-624.
80. Houghton DE, Vlazny DT, Casanegra AI, et al. Bleeding in patients with gastrointestinal cancer compared with nongastrointestinal cancer treated with apixaban, rivaroxaban, or enoxaparin for acute venous thromboembolism. Mayo Clin Proc. 2021;96(11):2793-2805.
81. Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021;5(4):927-974.
82. Kalaitzopoulos DR, Panagopoulos A, Samant S, et al. Management of venous thromboembolism in pregnancy. Thromb Res. 2022;211:106-113.
83. Cohen H, Arachchillage DR, Middeldorp S, et al. Management of direct oral anticoagulants in women of childbearing potential: Guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(8):1673-1676.
84. Barbhaiya M, Erkan D. Primary thrombosis prophylaxis in antiphospholipid antibody-positive patients: Where do we stand? Curr Rheumatol Rep. 2011;13(1):59-69.
85. Pabinger I, Thaler J. How I treat patients with hereditary antithrombin deficiency. Blood. 2019;134(26):2346-2353.
86. Middeldorp S, Nieuwlaat R, Baumann Kreuziger L, et al. American Society of Hematology 2023 guidelines for management of venous thromboembolism: Thrombophilia testing. Blood Adv. 2023;7(22):7101-7138.
87. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392.
88. White RH, Brunson A, Romano PS, et al. Outcomes after vena cava filter use in noncancer patients with acute venous thromboembolism: A population-based study. Circulation. 2016;133(21):2018-2029.
89. Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism: A randomized clinical trial. JAMA. 2015;313(16):1627-1635.
90. Li X, Haddadin I, McLennan G, et al. Inferior vena cava filter — comprehensive overview of current indications, techniques, complications and retrieval rates. Vasa. 2020;49(6):449-462.
91. Muriel A, Jiménez D, Aujesky D, et al. Survival effects of inferior vena cava filter in patients with acute symptomatic venous thromboembolism and a significant bleeding risk. J Am Coll Cardiol. 2014;63(16):1675-1683.
92. PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: The PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416-422.
93. Stein PD, Matta F. Vena cava filters in unstable elderly patients with acute pulmonary embolism. Am J Med. 2014;127(3):222-225.
94. Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med. 2012;125(5):478-484.
95. Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent post-thrombotic syndrome: A randomised placebo-controlled trial. Lancet. 2014;383(9920):880-888.
96. Subbiah R, Aggarwal V, Zhao H, et al. Effect of compression stockings on post thrombotic syndrome in patients with deep vein thrombosis: A meta-analysis of randomised controlled trials. Lancet Haematol. 2016;3(6):e293-300.
97. Rook B, van Rijn MJE, Jansma EP, van Montfrans C. Effect of exercise after a deep venous thrombosis: A systematic review. J Eur Acad Dermatol Venereol. 2024;38(2):289-301.
98. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e152S-e184S.
99. Stevens SM, Woller SC, Baumann Kreuziger L, et al. Executive Summary: Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160(6):2247-2259.
100. Li M, Li J, Wang X, et al. Oral direct thrombin inhibitors or oral factor Xa inhibitors versus conventional anticoagulants for the treatment of pulmonary embolism. Cochrane Database Syst Rev. 2023;4(4):CD010957.
101. Wang X, Ma Y, Hui X, et al. Oral direct thrombin inhibitors or oral factor Xa inhibitors versus conventional anticoagulants for the treatment of deep vein thrombosis. Cochrane Database Syst Rev. 2023;4(4):CD010956.
102. van der Hulle T, Kooiman J, den Exter PL, et al. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: A systematic review and meta-analysis. J Thromb Haemost. 2014;12(3):320-328.
103. Durrington C, Hurdman JA, Elliot CA, et al. Systematic pulmonary embolism follow-up increases diagnostic rates of chronic thromboembolic pulmonary hypertension and identifies less severe disease: Results from the ASPIRE Registry. Eur Respir J. 2024;63(3):2300846.