
Emerging Infectious Diseases
November 1, 2025
By Matthew Turner, MD, and Catherine Marco, MD, FACEP
Executive Summary
- Emerging infectious diseases are defined as infectious diseases that either are new to a specific population or are rapidly increasing in both incidence and geographic range.
- The majority of emerging infectious diseases are zoonotic, originating from wild animal reservoirs.
- Emerging infectious diseases may possess a high case-fatality rate, up to 90% with Marburg virus.
- Avian influenza should be considered if the patient has had a recent exposure to poultry or domestic birds.
- Mpox is spread by direct, prolonged contact with infected lesions or bodily fluids.
- The majority of individual infected with Crimean-Congo hemorrhagic fever virus have a mild or even asymptomatic course; only one in five patients develops the hemorrhagic stage.
- The majority of Oropouche virus infections are asymptomatic, and even cases of severe infection recover fully.
- Marburg virus is a highly virulent pathogen with a case fatality rate approaching 90%.
- Dengue virus is widespread in all major tropical areas across the globe.
- There are three forms of plague. Bubonic is the most common, followed by septicemic and then the least common, pneumonic plague.
Introduction
Emerging infectious diseases (EIDs) may be defined as infectious diseases that are either new to a specific population or that are rapidly increasing in both incidence and geographic range. Accounting for 15% of all human pathogens, the majority of EIDs are zoonotic, originating from wild animal reservoirs.1 As the front line for healthcare, emergency physicians should be aware of the potential threats developing around the globe, as well as the constant threat that EIDs pose to public health.2 This article will detail seven of the most threatening EIDs in terms of increasing spread, severity, and lethality.
Avian Influenza
Avian influenza, also known as “bird flu”, belongs to the genus Orthomyxoviridae and covers a range of Influenza A viruses that use wild birds as both a reservoir and a means of transmission.3,4 (See Figure 1.) Among the multiple subtypes, H5, H7, and H9 have spread to domestic poultry; H5 and H7 have successfully spread to humans as well, posing a significant threat to public health.5 The H5N1 subtype recently has spread to the Americas and has been detected in multiple non-avian species, raising concerns about its potential for future pandemics.6
Figure 1. Transmission of Avian Influenza |
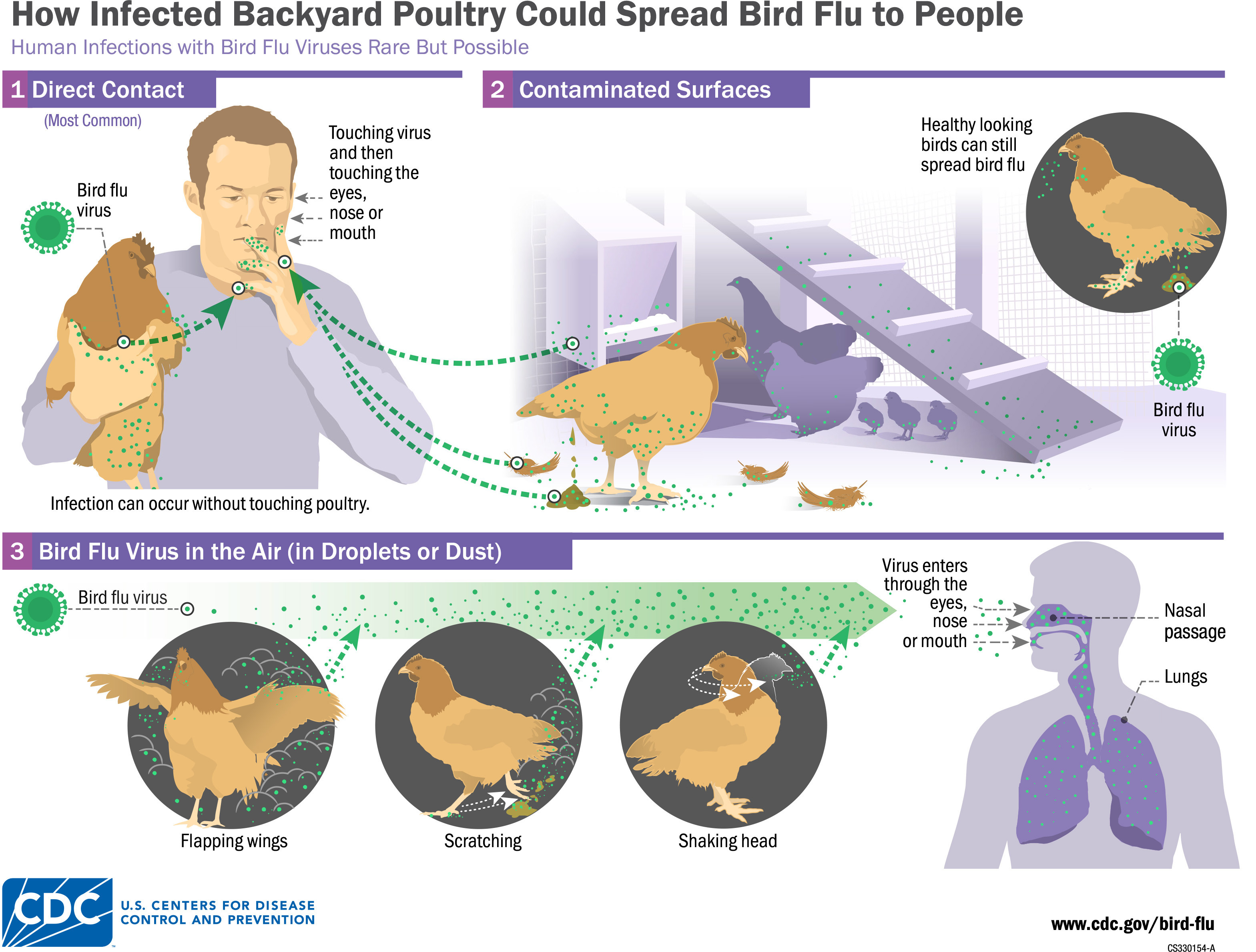 |
Source: Centers for Disease Control and Prevention |
Multiple outbreaks from various strains of avian influenza have occurred across the world in recent years. Examples include the H5N1 virus outbreak that caused the loss of nearly 400 million poultry across the globe from 2005-2022. In addition to the massive economic burden caused by these viruses, there is a significant human cost; more than 1,000 human deaths have been due to various strains of avian influenza.5 Avian influenza virus is exceptionally lethal, with fatality rates ranging from 50% to 80% in humans.7
Clinical Presentation
Human-to-human transmission of avian influenza remains rare, with the majority of cases occurring after exposure to infected poultry.8 The incubation period after exposure typically is two to five days.9 Initially, the infection will present as an uncomplicated influenza infection, with myalgias, upper respiratory symptoms, and fever.9 However, in severe cases, the infection will progress into a lower respiratory tract infection. In some cases, up to 90% of admitted patients will experience pneumonia or complete respiratory failure.10 Acute respiratory distress syndrome (ARDS) often manifests within five to seven days after the onset of symptoms.4 Further complications include encephalitis, septic shock, and multi-organ failure, including kidney failure, heart failure, and coagulopathies.9 Cases of encephalitis secondary to avian influenza, without any presentation of respiratory symptoms, also have been reported.9
Diagnosis
In cases of suspected avian influenza, a careful history should be obtained, with particular attention to any recent exposure to poultry or domestic birds.11 Patients presenting with a fever or any close contacts to confirmed cases of avian influenza also should be screened via a standard influenza rapid test.4 Polymerase chain reaction (PCR) testing should be conducted to identify the presence of avian influenza.4 Complete blood count (CBC) should be obtained to identify potential lymphocytopenia and thrombocytopenia.4
Treatment and Prevention
Older patients with multiple comorbidities are more at risk for severe avian influenza infections, while younger and healthy patients are more likely to have a milder clinical course or may be completely asymptomatic.4
Most laboratory-confirmed cases of influenza A, particularly the H7 subtype, have a high mortality rate, up to 50%. Thus, early initiation of antiviral treatment in patients with suspected avian influenza is imperative.11 In patients presenting with fevers and a recent confirmed exposure to avian influenza, antivirals may be initiated before confirmatory testing has resulted.4 Oseltamivir may be given as an antiviral; there is evidence that it halts the propagation of multiple strains of avian influenza and, thus, minimizes severe outcomes.12,13 Similarly, close contacts of avian influenza also may be prescribed oseltamivir 75 mg daily or zanamivir 10 mg twice daily for five to 10 days.4
Patients with suspected avian influenza should wear N95 masks or other respirators to minimize the spread of viral particles and practice thorough handwashing. In mild cases, rest and plenty of fluids should be strongly emphasized.4 Because of the potential for severe complications, more clinically concerning cases or cases in elderly patients with multiple comorbidities should be admitted for inpatient management and monitoring.4
Mpox
Mpox, previously known as monkeypox, was originally discovered in a primate research facility in Denmark in 1958.14 A double-stranded zoonotic deoxyribonucleic acid (DNA) virus in the Orthopox genus, mpox cases outside of Africa were exceptionally rare prior to 2022.15 Mpox came to global attention during the 2022 outbreak, where mpox infections were reported across 50 countries.16 Although the 2022 outbreak peaked in August and subsequently waned, mpox continues to have “outbreaks of increasing size and severity” in endemic areas within Western and Central Africa.17 At the time of this writing in late 2025, an outbreak has been reported in California, with at least nine reported cases, three of which occurred in patients with no recent travel.18
As noted earlier, mpox was originally identified in captive primates. This virus has a significant zoonotic reservoir that includes small rodents and other mammals.17 Mpox can be divided into two distinct genetic lineages: Clade I, which is endemic to Central Africa and often has a more severe clinical presentation, and Clade II, which is endemic to West Africa and generally milder.15 The large 2022 outbreak was primarily Clade II, although current reports of the disease in California show that is of the Clade I lineage.15,18 Mpox is closely related to smallpox and often presents as a smallpox-like disease; in the past, it is possible that it often was misdiagnosed as smallpox before that disease’s eradication, partially explaining its increasing incidence in recent years.17
While mpox is a zoonotic orthopoxvirus, it primarily is transmitted in human-to-human direct contact.15 Other forms of transmission may include bodily fluids, lesions, and fomites.16 Direct, prolonged contact with infected lesions or bodily fluids appears to the primary driver of human-to-human transmission.15 The 2022 outbreak was primarily seen in men who have sex with men (MSM).17 During the summer of 2022, more than 33,000 cases and 60 deaths occurred in the United States, largely in this population.15 However, it is important to emphasize that anyone is at risk for the transmission of mpox. In addition to this, Clade I infections are more likely to be driven by an initial zoonotic exposure, followed by spread of the disease within the affected household.15
Clinical Presentation
Mpox has a wide clinical presentation, ranging from a self-limiting illness to a life-threatening infection.15 Incubation ranges from two to 21 days after the initial exposure, but was as short as seven to 10 days in the 2022 outbreak.15 Symptoms initially will present with a nonspecific prodrome of chills, general malaise, lymphadenopathy, cough, headache, and fever.15,16 Early symptoms during the 2022 Clade II outbreak also included proctitis and pharyngitis.15 The patient should be considered infectious from the onset of their symptoms.15 Up to 98% of cases will develop a rash.16 Mpox presents chiefly as painful skin lesions that uniformly progress from macules to papules, then to vesicles, and finally to pustules over approximately two to four weeks.15 (See Figure 2.) Infections during the 2022 outbreak often presented with painful pustules in the anogenital areas, with up to 40% of patients displaying associated mucosal involvement.19 Eventually, the pustules will crust over. The patient may be considered no longer contagious once all the scabs have fallen away, with a fresh layer of skin now present.20
Figure 2. Mpox Lesions |
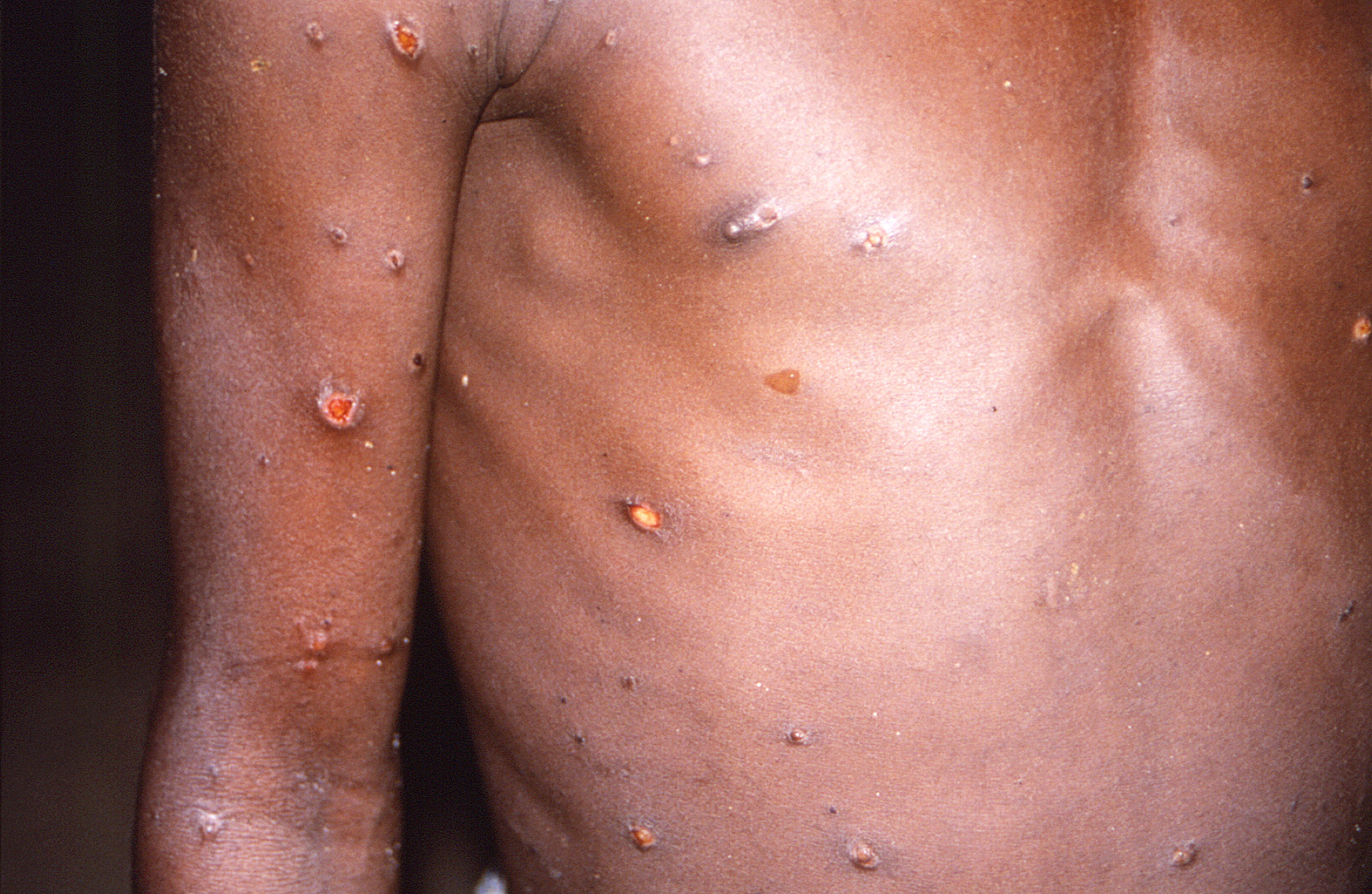 |
Source: Centers for Disease Control and Prevention/Brian W.J. Mahy, BSc, MA, PhD, ScD, DSc |
Further complications may arise during an mpox infection; these patients are susceptible to superimposed bacterial infections, sepsis, bronchopneumonia, and encephalitis.20 Immunocompromised individuals, such as those with human immunodeficiency virus (HIV) or organ transplants, are particularly at risk for severe complications.15 Fetal demise and preterm delivery also are possible in pregnant women.16
Diagnosis
Diagnosis may be made through viral PCR taken from a swab of the patient’s skin lesions, with at least two samples taken from different sites.15 Since 2023, the Food and Drug Administration (FDA) has authorized several point-of-care PCR tests to allow for rapid diagnosis.15
The differential diagnosis for mpox includes other poxviruses, such as chickenpox, molluscum contagiosum, and smallpox, as well as other rashes including scabies, syphilis, bacterial infections, and inflammatory diseases, among others.20
Treatment and Prevention
Mpox severity may be determined clinically by the number of lesions present, since they are proportional to the degree of fever, severity of other symptoms, and overall duration of illness that the patient will experience. If the patient has no more than 25 lesions, they will require no special care, while patients with 25-99 lesions should require nursing care, and those with 100 lesions or more will require intensive medical care.20 Of note, the majority of patients will experience mpox only as a self-limiting illness, with a full recovery within two to four weeks.15 In these cases, supportive care, such as covering rashes to prevent disease spread, wearing face gloves and masks, and receiving plenty of oral fluids in conjunction with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) to treat fever and pain, is enough.16 In cases of intractable pain, gabapentinoids and short courses of oral opioids also may be considered.15
In cases where there is concern for superimposed bacterial infections of mpox lesions, topical/systemic antibiotics also should be considered.15 While there are currently no FDA-approved treatments for mpox, a number of antiviral agents, including tecovirimat and cidofovir, have shown mixed off-label results.15
In more severe cases, patients may develop ocular involvement, with partial or even complete visual loss. An ophthalmologist should be consulted in cases where there is concern for visual impairment. Other unusual complications include the development of urethral and gastrointestinal strictures and scarring, both of which warrant evaluation by their respective specialties.15
Prevention should be strongly emphasized with vulnerable patient populations. Those of the MSM population, including gay, bisexual, and other men who have sex with men, have been shown to decrease their risk of transmission by reducing both the number of sexual partners and frequency of sexual encounters.15
Other prevention includes the possibility of vaccination. Of note, the substantial antigenic similarity between mpox and smallpox means that smallpox vaccination appears to offer a degree of protection against mpox.17 There are multiple reports of patients who had received the smallpox vaccination having much less severe infections, often presenting with only a single lesion.19 Unfortunately, with the eradication of smallpox and the end of large-scale vaccination programs, this means that there has been a significant increase in the population of susceptible individuals.17 The FDA recommends the ACAM2000 vaccination, a single dose smallpox vaccine, or the JYNNEOS double-dose vaccine against both smallpox and mpox, which became commercially available in the United States in April 2024.15,16
Crimean-Congo Hemorrhagic Fever
Reports of a mysterious tickborne disease similar to Crimean-Congo hemorrhagic fever (CCHF) go back to the 12th century, but the disease was not formally identified until 1945, when Soviet soldiers stationed in Crimea began to fall ill.21 In large part, CCHF has been defined by its extensive geographic distribution, as it has been identified across Africa, the Middle East, and Eastern Europe, thus earning its name.21
CCHF is a tickborne disease, with the disease transmitted through tick bites, crushing infected ticks, contact with patients in the acute stages of infection, or transmission of infected blood and tissue.21 Recent studies suggest that vertical and sexual transmission in humans may be possible.22 A member of the Bunyaviridae viral family, CCHF is a ribonucleic acid (RNA) arbovirus that is primarily transmitted by Hyalomma ticks across a wide geographic distribution. Interestingly, only humans are affected; all other species are asymptomatic.23 As a result, workers such as veterinarians, slaughterhouse workers, and farmers exposed to asymptomatic reservoirs including livestock, reptiles, and ostriches are at a significant risk of infection.21,24 While CCHF is endemic across much of Africa and Eurasia, the disease’s incidence is spreading; since 2000, CCHF has spread to nine new African countries and 11 new Asian countries.24 In recent years, CCHF has begun to spread into Western Europe through both animal trade and avian routes.22 It is currently classified as a Category C bioterrorism/biological warfare agent because of its potential for production and massive dissemination, and it is considered by the World Health Organization (WHO) to be one of the most important emerging infectious diseases, with a significant pandemic potential.22
Clinical Presentation
The majority of CCHF cases are mild or even asymptomatic, with patients only experiencing nonspecific symptoms including myalgias and arthralgias, headache, nausea, and vomiting.24 Approximately one in five patients infected will develop CCHF.21
CCHF has an incubation period of approximately two to 14 days following a tick bite or other exposure. Afterward, the patient will develop the pre-hemorrhage period, which is marked by sudden fevers, myalgias, headache, and dizziness. The patient also may develop hyperemia of the upper body, as well as conjunctivitis and congested sclera. The pre-hemorrhage period is rapid, and only lasts an average of three days.21
Next the patient goes into the hemorrhagic period. This period lasts only two to three days, but it is the most lethal; CCHF mortality ranges from 3% to 30%.21 This period is marked by severe and rapid hemorrhages, ranging from petechiae to large hematomas on the skin and mucus membranes.21 The trunk and the limbs appear to be the most common sites of this rash.25 The patient also will experience hematemesis, melena, epistaxis, hematuria, hemoptysis, menometrorrhagia, and even the possibility of cerebral hemorrhage since they have the potential to experience significant bleeding in nearly every organ system.21 Hematemesis and melena are associated with a higher incidence of death. Patients also may display marked hepatomegaly and splenomegaly during this period.21 Severe cases may develop hepatorenal failure, requiring dialysis, among other complications, secondary to multiorgan failure.26 Death resulting from cerebral herniation from cerebral edema also has been noted in the literature.26
If the patient survives the hemorrhagic period, they will then have a nine- to 10-day period of convalescence. This is marked chiefly by tachycardia, a labile pulse, polyneuritis, reported shortness of breath, and even cases of amnesia.21 Although there have been reports of re-infection and even a biphasic course of the disease, this appears to occur only in a minority of cases.21 Follow-up is not generally recommended for these cases.21
Diagnosis
CCHF has a similar presentation to other hemorrhagic viruses, such as Ebola and dengue.25 Differential diagnoses also may include rickettsia, Q fever, brucellosis, tick-borne encephalitis, malaria, and Marburg virus.21 In cases of suspected CCHF, a thorough patient history should be obtained, with a careful emphasis on any recent travel to known endemic areas, recent tick exposure, exposure to potentially asymptomatic animals, or recent outdoor activities.21
Laboratory tests in the early stages of the disease may show elevated creatine kinase, likely indicating muscle damage secondary to the myositis that patients experience.25 Patients also will consistently display thrombocytopenia, with the degree of thrombocytopenia directly associated with the severity of the disease. For example, while overall mortality is about 40%, patients with a platelet count below 20,000 have an estimated 90% fatality rate.21 Patients often display elevated levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), as well as prolonged coagulation studies, including prothrombin time and activated partial thromboplastin time.21
Diagnosis of the virus also may made done through reverse transcriptase PCR. A number of real-time assays for the diagnosis of CCHF also may be employed for even faster results.21 ELISA and immunofluorescence assays also may be used to detect immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies for up to seven days after the onset of disease and to detect IgG levels for up to five years after the disease.21
Treatment and Prevention
Nosocomial infection is dangerously common in cases of CCHF.26 In cases of possible CCHF, healthcare workers should immediately isolate the patient and use thorough barrier precautions, including gloves, gowns, face-shields, and goggles with side shields, to minimize the risk of transmission of the disease through infected fluids.21 Airborne precautions also should be used.26 Any healthcare workers possibly exposed to the virus should be followed up with CBC and routine biochemical tests for 14 days after their last contact with the patient.21 Ribavirin may be given to healthcare workers for postexposure prophylaxis.26
Supportive therapy should be the mainstay of treatment. The patient’s CBC should be checked at least once or twice per day and guide the administration of thrombocytes, fresh frozen plasma, and packed red blood cell repletion.21 In the hemorrhagic phase, the multiple foci of bleeding should be treated, such as avoiding NSAIDs and administering pantoprazole for gastrointestinal (GI) bleeding.21,27 The patient’s fluid and electrolyte status should be carefully monitored.21 Multiorgan failure may occur, and patients may require dialysis.26
Ribavirin is the recommended antiviral agent for treatment of CCHF, although its mechanism of action remains unclear.21 Although its use in treatment is somewhat controversial, it has been shown to significantly reduce mortality in animal models.21,22 While mild cases of CCHF do not require ribavirin, severe cases should be treated for 10 days, with 30 mg/kg as an initial loading dose, then 15 mg/kg every six hours for four days, and then 7.5 mg/kg every eight hours for six days.21 Ribavirin is associated with hypocalcemia, hypomagnesia, and hemolytic anemia, so these levels all should be carefully monitored.21 As noted earlier, oral ribavirin should be given to healthcare workers at a high risk of infection, typically through exposure to the patient’s blood or through needlestick injury.21 However, exposed healthcare workers also may simply be monitored via daily complete blood counts for a minimum of 14 days after exposure, with oral ribavirin only given if they develop a fever.21
While prior vaccines have been developed against CCHF, they are extremely limited both in production and in the duration of protection that they offer. Prevention in the form of personal protection against ticks, including thick clothing and repellents, as well as careful exposure when dealing with potentially infected livestock and animals, should be strongly emphasized, especially in endemic regions.21
Oropouche Virus
First identified in 1955, Oropouche virus (OROV) is another arthropod-borne virus of the Bunyaviridae family.28 This negative-sense, single-stranded RNA virus was first identified in Trinidad and Tobago, and is currently endemic throughout Central America, South America, and the Caribbean.29,30 As of March 2024, more than 500,000 cases of OROV have been identified, although no deaths and no human-to-human transmission have ever been confirmed.29 OROV has significantly increased in recent years. In 2024, its incidence in Brazil was 58.8 times greater than the annual median from 2015-2023, and it has had an “unprecedented” spread across Latin America since.31,32 Most concerning of all, there have been reports of several deaths possibly associated with this outbreak, suggesting that OROV may be worsening in severity.33
OROV is transmitted primarily through the biting midge Culicoides paraensis, particularly in urban settings, although it also may be transmitted by mosquitoes.29,30 Consequently, it is most prominent during the rainy seasons as its vectors reproduce.28 Climate change, which further spreads the virus’s vectors, as well as concerns over the virus’s increasing severity and potential for vertical/central nervous system (CNS) transmission, have led the WHO to determine that OROV has a concerning potential for future outbreaks.34
Clinical Presentation
Approximately 60% of OROV infections are symptomatic.30 The incubation period lasts an estimated four to eight days and is followed by an acute febrile illness that typically lasts two to seven days.28,29 Patients often experience myalgias, arthralgias, photophobia, headache, and rashes that may imitate rubella, in addition to reports of retro-orbital discomfort.28,29 (See Figure 3.) In severe cases, patients may develop neurological involvement, including meningitis, encephalitis, and Guillain-Barré syndrome.30 As with similar diseases such as Zika, vertical transmission is possible, increasing the risk of miscarriage in pregnant patients, as well as congenital deformities, including microcephaly.30-32 Mild epistaxis, gingival bleeding, and petechiae also may occur.29 However, even severe cases of the disease display no chronic sequelae, and convalescence usually is complete; there are no records of any fatalities that can be conclusively tied to OROV infection.28
Figure 3. Oropouche Virus Symptoms |
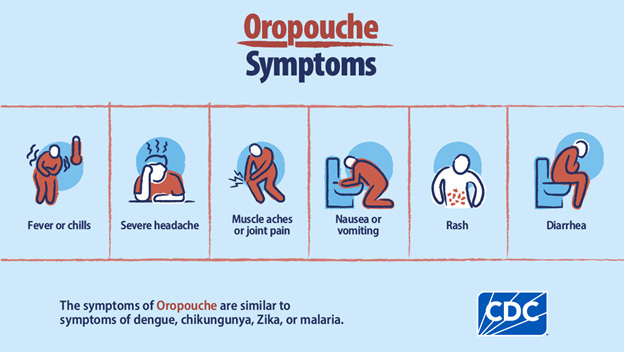 |
Source: Centers for Disease Control and Prevention |
Up to 60% of patients will display repeat symptoms one to two weeks later, giving OROV a unique biphasic clinical course.29 The most common reported symptoms are headache and fatigue.35
While OROV generally is considered a mild and self-limiting disease, there have been reports of two Brazilian patients dying from severe coagulopathy and liver failure secondary to the disease, in March 2024 and June 2024, respectively.33 OROV is difficult to monitor in South America because of a combination of political instability and poor surveillance; it remains to be seen if these cases were isolated events, misdiagnosed, or an indication of the virus’s increasing severity.33 Regardless, OROV is rapidly becoming a significant threat to public health within South America.36
Diagnosis
It is likely that OROV is significantly underreported because of its high degree of clinical similarity to illnesses such as dengue, West Nile virus, yellow fever, and Zika.29 As a self-limited, nonspecific disease, it often can be difficult to diagnose.31 Patients often will display thrombocytopenia, leukopenia, and elevated liver enzymes during the acute stage of the disease.33 Patients with CNS involvement also may have detectable viral RNA present in their cerebrospinal fluid.33
PCR testing is the gold standard for diagnosing OROV; PCR may detect OROV in urine and whole blood samples up to 22 days after symptom onset. Serological IgM tests to assess for antibodies against OROV also may be employed four to five days after symptom onset.34 Unfortunately, there are no commercial tests or point-of-care testing currently available.34
Treatment and Prevention
Treatment for OROV should focus on symptomatic management, since there are no specific antiviral medications available for the treatment of this disease. Hydration, analgesics, and antipyretics should be employed, while patients with more severe symptoms should be admitted to the hospital for close monitoring.36
There are currently no vaccines available for OROV, although research is ongoing.37 Prevention should focus on vector control and minimizing the potential for insect bites, particularly in pregnant women who are most at risk for vertical transmission.35
Marburg Virus
As a member of the Filovirus family, Marburg virus (MARV) is a single-stranded, negative-sense RNA virus.38 It was first identified in Marburg, Germany, in 1967 in monkeys imported from Uganda, earning it its name.38 Since then, it has had notable outbreaks in sub-Saharan Africa, where it is endemic, as well as an outbreak in Russia in the 1990s following a laboratory accident.39 MARV is exceptionally lethal and, thus, is considered one of the “most devastating and virulent pathogens that affect humans.”39 It has a fatality rate approaching 90%, with no currently available vaccinations and no established management course.39 More research into MARV is needed, since there have been recent outbreaks of the disease in Equatorial Guinea and Tanzania in 2023 alone.40
MARV’s natural reservoir is fruit bats in caves and mines. One significant outbreak in the Democratic Republic of the Congo from 1998-2000 chiefly involved local gold miners.39 Human-to-human transmission also is possible, chiefly through transmission of bodily fluids. Transmission also may occur from consumption of infected bushmeat and exposure to aerosols.41 Often, this means that family members of the infected patients and healthcare workers are at the greatest risk of infection.41
Clinical Presentation
MARV has an incubation period ranging from two to 21 days.42 Early symptoms generally are nonspecific and include myalgias, abdominal pain, nausea, and vomiting, as well as persistent watery diarrhea that may last up to a week.42,43 Other systemic symptoms include fever, headache, fatigue, and conjunctivitis.43 Interestingly, patients during this period often are characterized by sunken eyes, severe fatigue, and “indifferent facial expressions.”42 Patients also may display leukopenia and thrombocytopenia.43 Patients may display a characteristic maculopapular rash that distinguishes them from an influenza or malarial infection.44
Hemorrhagic signs typically appear five to seven days after the initial symptoms develop.42 This second phase of the disease typically lasts for five to 13 days following the initial onset of symptoms.43 Approximately 75% of patients will experience hemorrhagic symptoms, including hematemesis, bloody diarrhea, epistaxis, bleeding from the gums, hematuria, petechiae, and even subconjunctival hemorrhage.43 Patients often develop bleeding at intravenous (IV) sites.42 This severe phase of MARV also is marked by high fevers and confusion.42 Death usually occurs approximately eight to nine days after the onset of disease, typically secondary to hemorrhagic shock.42 The overall case fatality rate is approximately 72%.43
If the patient survives the hemorrhagic phase, they will enter a protracted convalescence phase that may last up to another week. During this period, the patient is susceptible to metabolic abnormalities and dehydration, raising the risk of multiple organ failures as well as orchitis in male patients.43 Other secondary infections also are common.44 Patients may develop significant sequelae, including amnesia, skin desquamation, myalgias, and exhaustion. There are accounts of patients transmitting the virus through intercourse more than two months after recovery.38
Diagnosis
MARV often is seen in rural and impoverished regions, making it difficult to work up clinically.39 However, enzyme-linked immunosorbetn assay (ELISA) testing for viral antigens may be performed during the early stages of the disease, as well as reverse transcriptase PCR in both the early and late stages of the disease.41 Patients may display a characteristic thrombocytopenia, leukopenia, elevated liver enzymes, and various abnormalities in the coagulation pathways.41 Patients also often display large and abrupt changes in their body temperature, ranging from hyperpyrexia to hypopyrexia.38
Treatment and Prevention
Because of the risk of nosocomial transmission, patients should be carefully evaluated for the possibility of viral hemorrhagic fevers on initial presentation, including a detailed travel history. In cases where infectious causes such as MARV are suspected, patients should be isolated, with appropriate personal protective equipment (PPE) employed by healthcare workers.41
MARV is one of the deadliest human pathogens known, and currently no approved treatments are available.44 Supportive care, including fluids, blood transfusions, and the use of antibiotics to treat secondary infections, currently are the standard of care.44 Palliative medications to ease patient’s pain, as well as careful monitoring of blood volume and the patient’s electrolyte balance, also should be employed.39 There is some early evidence to suggest that remdesivir may be helpful when given early in the infection, but more research is required.39
Currently, there are no vaccines available for MARV.45 Although the viruses are closely related, there is no cross protection between Ebola vaccines and MARV.38
Family members potentially exposed to MARV should quarantine and monitor themselves for 21 days, with instructions to immediately seek medical care if they develop symptoms. Any exposures to mines or caves with a significant fruit bat presence should be avoided if possible, and, during outbreaks, any close physical contact or contact with infected blood and fluids also should be avoided.41
Dengue Virus
Dengue virus (DENV) is an acute arthropod-borne virus that has been documented for at least 2,000 years.46,47 This single-stranded, positive-sense RNA virus belongs to the Flaviviridae family and infects anywhere between 50 million and 200 million people annually.47 DENV is primarily transmitted through the Aedes aegypti mosquito, often in crowded, unsanitary urban environments where vector management is minimal at best.47
DENV is considered the most important of the arboviruses that affect humans, since it occurs in all major tropical areas across the globe; more than 3.6 billion people live in areas that are at risk for infection.47 In Southeast Asia alone, there is an average of 3.3 infections per person per lifetime.47 Since the 1950s, DENV has dramatically expanded across Asia, the Pacific, and the Americas.47 Endemic countries have reported a 30-fold increase in cases since the 1960s. From 2000 to 2013, there was a reported 400% increase in incidence of DENV.46 The dramatic urban growth seen in endemic regions over the past decades, as well as the effects of climate change, likely will increase the incidence of DENV even further.47 Even the United States has seen an increase in DENV incidence in recent years — not only in territories such as Puerto Rico, where dengue is endemic, but also in Florida, Hawaii, and Texas, where recent outbreaks have occurred.48
Clinical Presentation
Dengue is a self-limiting febrile illness; of those infected, 60% to 80% of those infected either will be completely asymptomatic or will have only subclinical infections.49 After being bitten by an infected mosquito, the patient will undergo an incubation period that typically lasts five to seven days, although it may last up to two weeks.47
WHO guidelines previously classified dengue as dengue fever, dengue hemorrhagic fever, and dengue shock syndrome. However, the WHO revised its guidelines in 2009, and dengue now is classified as dengue without warning signs, dengue with warning signs, and severe dengue.49 Pregnant patients and older adult patients are at a higher risk for developing severe disease.49 Interestingly, older children and adults experiencing their second dengue infection are at the highest risk for severe disease because of the risk of exposure to a new serotype of DENV.48
Afterward, patients will enter the febrile phase. This phase typically lasts three to seven days and is marked by a rapid onset of a high fever (39°C to 40°C). Patients also will experience nonspecific symptoms, including arthralgias, myalgias, headache, nausea, and vomiting, as well as general malaise.47 During this period, patients may display facial erythema, conjunctival injection, a petechial rash, and generalized truncal erythema.47,49 Patients may experience minor bleeding in the form of petechiae, epistaxis, bruising at IV sites, hematuria, or melana.47,49 In cases of simple dengue fever, the patients’ fever and symptoms will resolve in a self-limited fashion.49
In severe cases, some patients will enter the critical phase from days 4 to 6 of the illness.49 The critical phase is characterized by a widespread vasculopathy, resulting in a massive increase in vascular permeability across the body.47 In worsening cases, patients will display severe abdominal tenderness, vomiting, extravascular fluid accumulation, hepatomegaly, and a sudden increase in hematocrit as their plasma volume precipitously drops.49 Cases of dengue with warning signs, per the WHO definition, fall into this category, displaying persistent vomiting, mucosal bleeds, liver enlargement greater than 2 cm, clinical fluid accumulation, lethargy, several abdominal pain, and laboratory evidence of hemoconcentration.49
In the most severe cases, also classified as severe dengue by the WHO, patients will experience a profound loss of plasma secondary to this increased vascular permeability, leading to fatal hypovolemic shock.47,49 If untreated, the mortality rate in these patients may be as high as 20%.50 Patients will display ascites, pleural effusions, and cardiovascular decompensation.47 The most important sign in this category is narrowing of the pulse pressure, typically to 20 or less, which indicates that the patient’s plasma depletion has reached a critical point and that the patient is on the precipice of decompensated shock and requires immediate fluid resuscitation.46,47 Interestingly, patients often may be clinically well-appearing at this time, despite being on the verge of decompensated shock.46 Further criteria for severe dengue include respiratory distress secondary to fluid accumulation or shock, severe bleeding, severe organ involvement including the CNS, heart, or liver.49
The critical phase is followed by the recovery phase. During this period, patients may display a characteristic “Herman’s rash” with white islands of unblemished skin separated by areas of erythema and pruritus. The majority of patients recover without issue, although fatigue and depression may persist for several weeks to months.49
Diagnosis
DENV initially presents as a nonspecific febrile illness, and thus has a broad differential diagnosis, including measles, rubella, influenza, typhoid fever, and malaria.49 In the febrile phase of the illness, patients often display mild thrombocytopenia and leukopenia, along with elevated liver function tests (LFTs).47 Lower platelet counts are more concerning with a progression to severe disease, as is an increasing hematocrit level, which indicates further plasma loss and hemoconcentration.49 Severe dengue often is characterized by a severe increase in LFTs, with AST or ALT at 1,000 IU/L or greater.49 In cases where there is concern over increasing vasculopathy, point-of-care ultrasound may be used to identify ascites, pleural effusion, and other evidence of plasma leakage.49
Nucleic acid amplification tests (NAATs) also can detect DENV RNA from blood samples within the first seven days of illness with a high degree of accuracy.48 Patients presenting more than seven days after symptom onset can have anti-DENV IgM testing done, although this method often cross-reacts with other flaviviruses such as Zika.48
Treatment and Prevention
There are no medications that currently demonstrate a significant reduction in the clinical presentation or complications of DENV. Treatment should focus on supportive care. While the majority of patients with uncomplicated dengue may be successfully treated in the outpatient setting, patients at risk for severe dengue should be managed in the inpatient setting.48 (See Figure 4.)
Figure 4. Dengue Case Management |
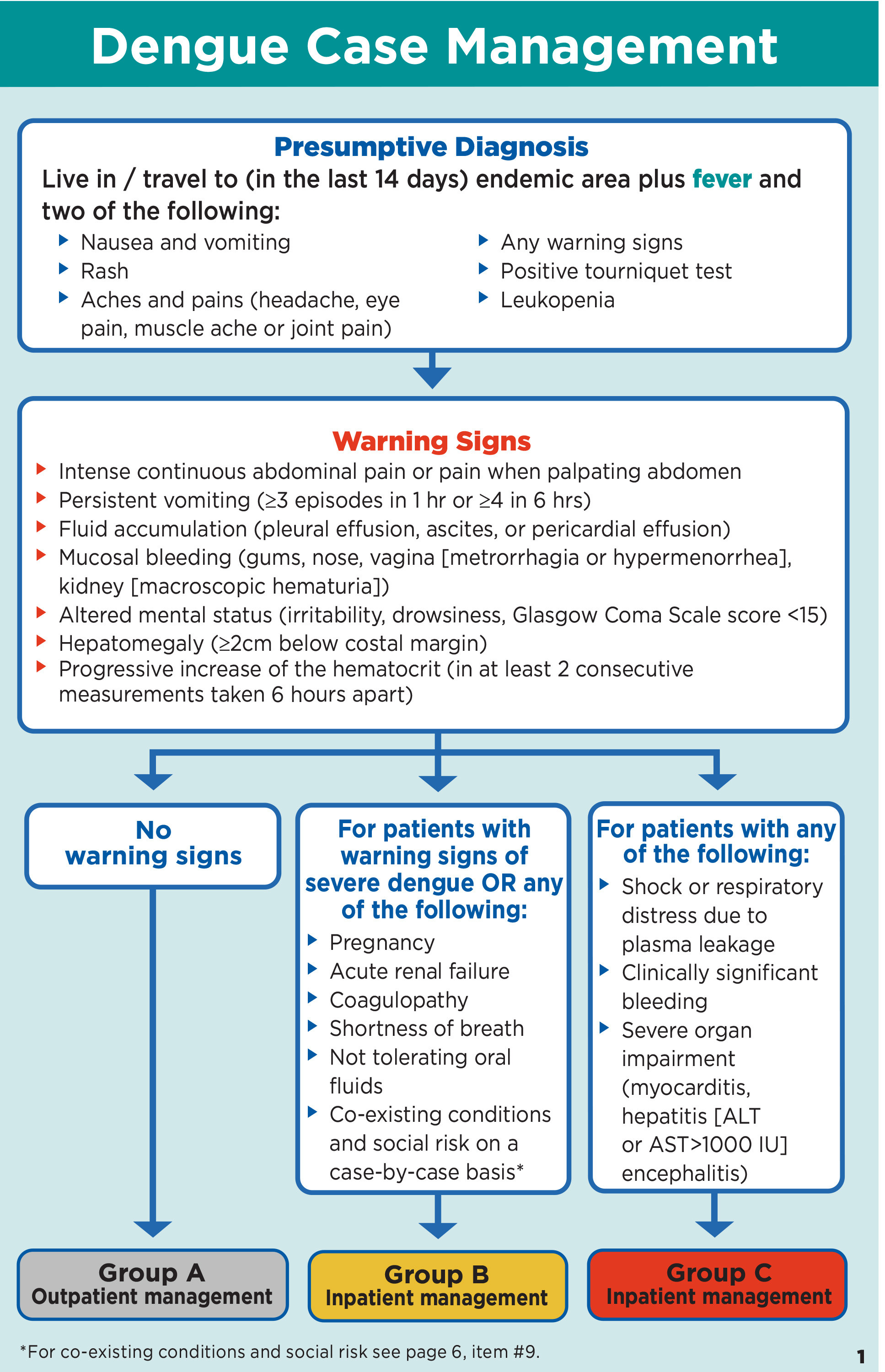 |
Source: Centers for Disease Control and Prevention |
Uncomplicated cases may be controlled with rehydration and fever management, including physical cooling measures and medications such as acetaminophen. NSAIDs should be avoided because of the risk of bleeding and thrombocytopenia.48 These patients may be discharged to home, provided they have no warning signs, and close follow-up.49 (See Figure 5.) Cases of dengue with warning signs and severe dengue should be managed with large-volume fluid resuscitation, with careful monitoring to avoid the potential for fluid overload.48 Steroids, immunoglobins, and prophylactic platelet transfusions have no demonstrated benefit.48 The mortality rate for severe dengue can be reduced to less than 1% with early diagnosis and appropriate management.48 (See Figure 6.)
Figure 5. Dengue Outpatient Management |
 |
Source: Centers for Disease Control and Prevention |
Figure 6. Dengue Inpatient Management |
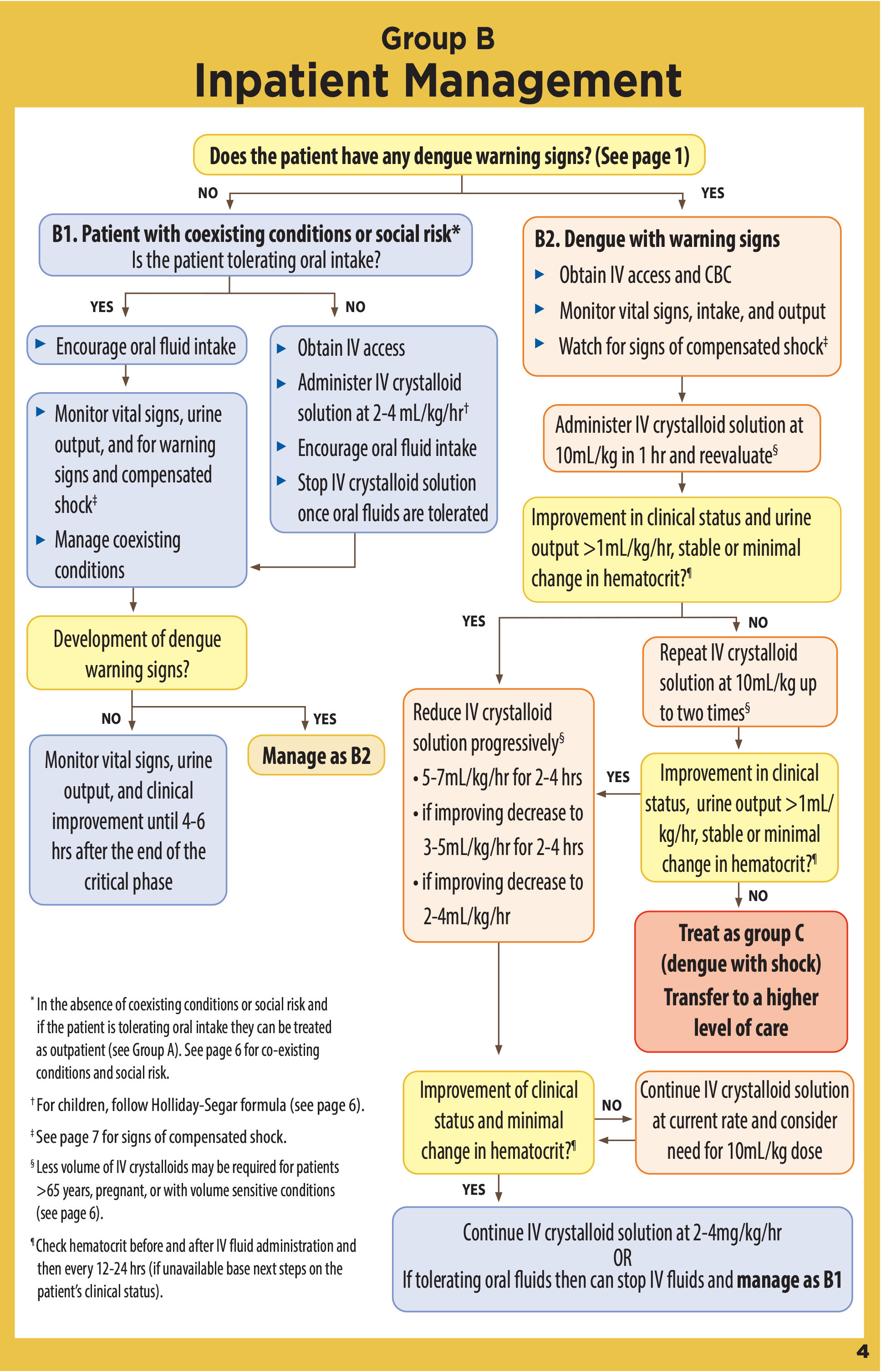 |
Source: Centers for Disease Control and Prevention |
A number of dengue vaccines, including Dengvaxia, Qdenga, and TV003, are available. All of these vaccines target each of the four different DENV serotypes.49 Vector control, such as the use of insect repellents, clothing that offers protection against mosquitos, bednets, and other anti-mosquito interventions, in dengue-endemic areas also should be strongly urged.49
Plague
Plague is one of the most infamous diseases to afflict humanity and has persisted in Eurasia for at least 5,000 years.51 The effect that plague has had on the course of human history cannot be overstated. The three major historical pandemics of plague include the Plague of Justinian from 541-549, the Chinese pandemic in the 19th century, and most infamously of all, the Black Death of 1347-1351, responsible for killing approximately 30% to 40% of the population in Europe.52 Notably, it also was employed as a biological warfare agent by Imperial Japan during the World War II.53 In the current day, plague is endemic to the United States, Madagascar, China, Mongolia, Russia, and large areas of Africa.52
Plague is a zoonotic disease caused by the gram-negative bacterium Yersinia pestis.51 Animal reservoirs include more than 200 mammals, chiefly rodents and lagomorphs,52 and transmission occurs largely through the bites of infected fleas.51 However, other forms of infection — through pneumonic plague or the consumption of contaminated raw meat — also may occur.51 Plague is currently considered a tier 1 bioterrorism agent because of its severity and epidemic potential,54 and remains endemic in more than 25 countries.55 Notably, two cases of it independently occurred in August 2015 from infected squirrels in Yosemite.56
Clinical Presentation
There are three distinct forms of the plague. Bubonic plague is the most common form, accounting for approximately 80% of U.S. cases.53 Patients will suddenly develop high fevers, severe abdominal pains, headaches, arthralgias, and myalgias three to seven days after an initial flea bite or after handling the tissue and bodily fluids of an infected animal.53,56 Patients may experience a localized lesion at the site of the bite.54 From there, the Yersinia pestis bacteria will infect and reproduce within the regional lymph nodes, creating the distinctive and exquisitely buboes of this disease.54 These buboes are extremely painful and may swell up to 10 cm. They are especially prone to suppuration and infection, and, in untreated cases of bubonic plague, approximately 60% of patients will die within one week of exposure.56 Clinical diagnosis in these cases is relatively easy; in addition to the characteristic buboes, patients will present with fever, hypotension, cough, chest pain, and severe fatigue.57 Unfortunately, even with proper treatment, mortality may be as high as 10%.58
The second form of the plague, septicemic plague, is responsible for 10% to 15% of plague cases. Rather than being limited to buboes, the Y. pestis bacteria reproduce within the blood, causing disseminated intravascular coagulation (DIC) in addition to extensive gangrene of the extremities, nose, and ears.56 Patients also may develop digital necrosis.53
Pneumonic plague is the least frequent form of the plague and the only one with the potential for human-to-human transmission. This plague presentation is transmitted by the inhalation of aerosolized droplets.56 Within four days of exposure to the aerosolized plague, patients will experience nonspecific, flu-like symptoms such as dyspnea and a rapid onset of high fevers; however, there are reports of the incubation period being as short as 24 hours.53,56 Patients then will rapidly develop a purulent, bloody cough, with evidence of a rapidly spreading lobar pneumonia.56 In these cases, the patient’s sputum is highly contagious.58 If left untreated, pneumonic plague is nearly always fatal, sometimes as rapidly as within 18-24 hours.53,55 Pneumonic plague is considered a highly dangerous candidate for bioterrorism; a recent 2017 outbreak in Madagascar resulted in more than 2,000 cases.55
Diagnosis
Bubonic plague often can be diagnosed clinically because of the characteristic exquisitely tender buboes that patients develop.54 Unfortunately, this means that septicemic plague and pneumonic plague, which often do not display buboes, may be more difficult to diagnose clinically.57 Patients who have developed a fever after handling dead rodents in an endemic zone, have buboes with fever and hypotension, or have pneumonia with hemoptysis and gram-negative bacilli in their sputum all should be considered extremely high-risk for possible plague.57 CBCs may show significant leukocytosis, with a white blood cell count above 20,000, and a concurrent thrombocytopenia. Chest X-rays often will be nonspecific.57
A number of diagnostic tests are available, including a rapid diagnostic test that may provide results within 15 minutes.55 PCR testing also may identify Y. pestis within several hours.55 However, microbial isolation of Y. pestis on cefsulodin-irgasan-novobiocin (CIN) agar for 48-72 hours remains the gold standard for diagnosis.55 Testing of suspected plague samples should be done only in a Biosafety Level 3 laboratory.55
In cases of pneumonic plague, bronchoalveolar lavage fluid may be tested for Y. pestis.59 Deep respiratory secretions are necessary for the diagnosis of pneumonic plague, meaning that saliva and spit cannot be tested.55
Treatment and Prevention
Early identification and treatment of plague is imperative. Without antibiotics, plague has an overall fatality rate as high as 66%.56 However, early diagnosis and antibiotic treatment can drastically reduce the mortality rate to as low as 5%.60 Thus, in suspected cases of plague, early treatment with antibiotics is essential.
There are several recommended antibiotic treatments for the plague, all of which should last for 10 days. Streptomycin, 1 gram twice per day (BID) IV or intramuscular (IM) is one of the standard treatments; other treatments include doxycycline 200 mg once followed by 100 mg BID orally (PO) or ciprofloxacin 750 mg BID PO.53,57,60 In cases of potential exposure, patients may be treated with a number of medications including ciprofloxacin, levofloxacin, doxycycline, or trimethoprim-sulfamethoxazole for postexposure prophylaxis.53 (See Table 1.)
Table 1. Medications for Treatment of Plague53 |
Ciprofloxacin/Levofloxacin
Moxifloxacin
Doxycycline
Streptomycin
Trimethoprim-Sulfamethoxazole
|
Treatment is typically 10-14 days, or two days after fever subsidence. Hospitalized patients are typically started on parental antibiotics and switched to oral antibiotics when their condition improves. BID: twice per day; PO: orally; IV: intravenous; IM: intramuscular |
Although vaccines against plague once were available, they since have been discontinued because of short-lasting protection and minimal efficiency. Research into vaccines against pneumonic plague is ongoing.55 On a population level, prevention should focus on careful surveillance of potential plague vectors and public education.53
Conclusion
In the modern globalized era, the threat of emerging infectious diseases looms large. It is imperative that emergency medicine physicians be aware of the growing threats that EIDs pose to public health.
Matthew Turner, MD, Milton S. Hershey Medical Center Emergency Department, Hershey, PA.
Catherine A. Marco, MD, FACEP, is Professor, Department of Emergency Medicine, Penn State Health, Hershey Medical Center, Hershey, PA.
References
1. McArthur DB. Emerging infectious diseases. Nurs Clin North Am. 2019;54(2):297.
2. Yaffee AQ, Isakov A, Wu HM. How can emergency departments better prepare for emerging infectious disease threats? A returned traveler with fever walks into triage... J Emerg Med. 2019;56(5):568-570.
3. Simancas-Racines A, Cadena-Ullauri S, Guevara-Ramírez P, et al. Avian influenza: Strategies to manage an outbreak. Pathogens. 2023;12(4):610.
4. Sivanandy P, Xien FZ, Kit LW, et al. A review on current trends in the treatment of human infection with H7N9-avian influenza A. J Infect Public Health. 2019;12(2):153-158.
5. Shi J, Zeng X, Cui P, et al. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg Microbes Infect. 2023;12(1):2155072.
6. Krammer F, Schultz-Cherry S. We need to keep an eye on avian influenza. Nat Rev Immunol. 2023;23(5):267-268.
7. Bell MA, Dake JA, Price JH, et al. A national survey of emergency nurses and avian influenza threat. J Emerg Nurs. 2014;40(3):212-217.
8. Philippon DAM, Wu P, Cowling BJ, Lau EHY. Avian influenza human infections at the human-animal interface. J Infect Dis. 2020;222(4):528-537.
9. Nuñez IA, Ross TM. A review of H5Nx avian influenza viruses. Ther Adv Vaccines Immunother. 2019;7:2515135518821625.
10. Li Q, Zhou L, Zhou M, et al. Epidemiology of human infections with avian influenza A (H7N9) virus in China. N Engl J Med. 2014;370(6):520-532.
11. Liao Q, Ip DKM, Tsang TK, et al. A clinical prediction rule for diagnosing human infections with avian influenza A (H7N9) in a hospital emergency department setting. BMC Med. 2014;12(1):127.
12. Yang Z-F, Mok CK, Peiris JS, Zhong N-S. Human infection with a novel avian influenza A (H5N6) virus. N Engl J Med. 2015;373(5):487-489.
13. Wei S-H, Yang J-R, Wu H-S, et al. Human infection with avian influenza A H6N1 virus: An epidemiological analysis. Lancet Respir Med. 2013;1(10):771-778.
14. Ulaeto D, Agafonov A, Burchfield J, et al. New nomenclature for mpox (monkeypox) and monkeypox virus clades. Lancet Infect Dis. 2023;23(3):273.
15. Titanji BK, Hazra A, Zucker J. Mpox clinical presentation, diagnostic approaches, and treatment strategies: A review. JAMA. 2024;332:1652-1662.
16. Mansoor A, Mansoor E, Waheed Y, et al. Update on the M-pox virus and safety measures taken against it globally. J Formos Med Assoc. 2024;123(10):1030-1036.
17. Van Dijck C, Hoff NA, Mbala-Kingebeni P, et al. Emergence of mpox in the post-smallpox era—a narrative review on mpox epidemiology. Clin Microbiol Infect. 2023;29(12):1487-1492.
18. Stone W. 3 cases of new mpox strain reported in California. NPR. Oct. 22, 2025. https://www.npr.org/2025/10/22/nx-s1-5580567/3-cases-of-new-mpox-strain-reported-in-california
19. Hazra A, Zucker J, Bell E, et al. Mpox in people with past infection or a complete vaccination course: a global case series. Lancet Infect Dis. 2024;24(1):57-64.
20. Ogoina D, Damon I, Nakoune E. Clinical review of human mpox. Clin Microbiol Infect. 2023;29(12):1493-1501.
21. Ergönül Ö. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6(4):203-214.
22. Portillo A, Palomar AM, Santibáñez P, Oteo JA. Epidemiological aspects of Crimean-Congo hemorrhagic fever in Western Europe: What about the future? Microorganisms. 2021;9(3):649.
23. Shayan S, Bokaean M, Shahrivar MR, Chinikar S. Crimean-Congo hemorrhagic fever. Lab Med. 2015;46(3):180-189.
24. Temur AI, Kuhn JH, Pecor DB, et al. Epidemiology of Crimean-Congo hemorrhagic fever (CCHF) in Africa—underestimated for decades. Am J Trop Med Hyg. 2021;104(6):1978.
25. Eslava M, Carlos S, Reina G. Crimean-Congo hemorrhagic fever virus: An emerging threat in Europe with a focus on epidemiology in Spain. Pathogens. 2024;13(9):770.
26. Conger NG, Paolino KM, Osborn EC, et al. Health care response to CCHF in US soldier and nosocomial transmission to health care providers, Germany, 2009. Emerg Infect Dis. 2015;21(1):23.
27. Yadav PD, Thacker S, Patil DY, et al. Crimean-Congo hemorrhagic fever in migrant worker returning from Oman to India, 2016. Emerg Infect Dis. 2017;23(6):1005.
28. da Rosa JFT, de Souza WM, de Paula Pinheiro F, et al. Oropouche virus: Clinical, epidemiological, and molecular aspects of a neglected orthobunyavirus. Am J Trop Med Hyg. 2017;96(5):1019.
29. Zhang Y, Liu X, Wu Z, et al. Oropouche virus: A neglected global arboviral threat. Virus Res. 2024;341:199318.
30. Pastula DM, Beckham JD, Tyler KL. Oropouche virus: An emerging neuroinvasive arbovirus. Ann Neurol. 2025;97(1):28-33.
31. Scachetti GC, Forato J, Claro IM, et al. Re-emergence of Oropouche virus between 2023 and 2024 in Brazil: An observational epidemiological study. Lancet Infect Dis. 2025;25(2):166-175.
32. Rodriguez-Morales AJ, Drexler JF. Re-emergence of Oropouche virus in Brazil and Latin America. Lancet Infect Dis. 2025;25(2):137-139.
33. Tilston-Lunel NL. Oropouche virus: An emerging orthobunyavirus. J Gen Virol. 2024;105(10):002027.
34. Lapa D, Romeo MA, Spina A, et al. Oropouche virus: An overview of the current status of diagnostics. Viruses. 2025;17(10):1382.
35. Porwal S, Malviya R, Sridhar SB, et al. Mysterious Oropouche virus: Transmission, symptoms, and control. Infect Med (Beijing). 2025;4(2):100177.
36. Yoosuf BT, Gaidhane AM, Vadia N, et al. Epidemiology, transmission dynamics, treatment strategies, and future perspectives on Oropouche virus. Diagn Microbiol Infect Dis. 2025;113(1):116882.
37. Vijukumar A, Kumar A, Kumar H. Potential therapeutics and vaccines: Current progress and challenges in developing antiviral treatments or vaccines for Oropouche virus. Diagn Microbiol Infect Dis. 2025;111(3):116699.
38. Kortepeter MG, Dierberg K, Shenoy ES, Cieslak TJ. Marburg virus disease: A summary for clinicians. Int J Infect Dis. 2020;99:233-242.
39. Abir MH, Rahman T, Das A, et al. Pathogenicity and virulence of Marburg virus. Virulence. 2022;13(1):609-33.
40. Cuomo-Dannenburg G, McCain K, McCabe R, et al. Marburg virus disease outbreaks, mathematical models, and disease parameters: A systematic review. Lancet Infect Dis. 2024;24(5):e307-e17.
41. Alla D, Paruchuri SSH, Tiwari A, et al. The mortality, modes of infection, diagnostic tests, and treatments of Marburg virus disease: A systematic review. Health Sci Rep. 2023;6(9):e1545.
42. Srivastava S, Sharma D, Kumar S, et al. Emergence of Marburg virus: A global perspective on fatal outbreaks and clinical challenges. Front Microbiol. 2023;14:1239079.
43. Pisapia R, Fusco FM, Scorzolini L, et al. Clinical features of Marburg virus disease: A review of all reported patients since 1967. eClinicalMedicine. 2025;89:103581.
44. Brauburger K, Hume AJ, Mühlberger E, Olejnik J. Forty-five years of Marburg virus research. Viruses. 2012;4(10):1878-927.
45. Albakri K, Al-Hajali M, Saleh O, et al. Marburg virus disease treatments and vaccines: Recent gaps and implications. Ann Med Surg (Lond). 2023;85(2):328-330.
46. Wilder-Smith A, Ooi E-E, Horstick O, Wills B. Dengue. Lancet. 2019;393(10169):350-363.
47. Murugesan A, Manoharan M. Chapter 16: Dengue virus. In: Emerging and Reemerging Viral Pathogens. Elsevier; 2020:281-359.
48. Wong JM, Adams LE, Durbin AP, et al. Dengue: A growing problem with new interventions. Pediatrics. 2022;149(6):e2021055522.
49. Paz-Bailey G, Adams LE, Deen J, et al. Dengue. Lancet. 2024;403(10427):667-682.
50. Khan MB, Yang Z-S, Lin C-Y, et al. Dengue overview: An updated systemic review. J Infect Public Health. 2023;16(10):1625-1642.
51. Barbieri R, Signoli M, Chevé D, et al. Yersinia pestis: The natural history of plague. Clin Microbiol Rev. 2020;34(1):e00044-19.
52. Vallès X, Stenseth NC, Demeure C, et al. Human plague: An old scourge that needs new answers. PLoS Negl Trop Dis. 2020;14(8):e0008251.
53. Johnson M, Gooch MD. From history books to headlines: Plague in modern times. Adv Emerg Nurs J. 2025;47(2):137-144.
54. Nelson CA, Fleck-Derderian S, Cooley KM, et al. Antimicrobial treatment of human plague: A systematic review of the literature on individual cases, 1937–2019. Clin Infec Dis. 2020;70(70 Suppl 1):S3-S10.
55. Demeure CE, Dussurget O, Mas Fiol G, et al. Yersinia pestis and plague: An updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics. Genes Immun. 2019;20(5):357-370.
56. Glatter KA, Finkelman P. History of the plague: An ancient pandemic for the age of COVID-19. Am J Med. 2021;134(2):176-181.
57. Raoult D, Mouffok N, Bitam I, et al. Plague: History and contemporary analysis. J Infect. 2013;66(1):18-26.
58. Randremanana R, Andrianaivoarimanana V, Nikolay B, et al. Epidemiological characteristics of an urban plague epidemic in Madagascar, August–November, 2017: An outbreak report. Lancet Infect Dis. 2019;19(5):537-545.
59. Yang J, Mei X, Zhao LX, et al. Emergency department experience in the multi-dimensional prevention and control of pneumonic plague in Beijing. Biomed Environ Sci. 2020;33(12):948-952.
60. Nikiforov VV, Gao H, Zhou L, Anisimov A. Plague: Clinics, diagnosis and treatment. Adv Exp Med Biol. 2016:293-312.