
Clinical Insights on Managing DPN and PAD in Patients with Diabetes
April 1, 2025
By Frank Lavernia, MD; Carrie O’Day, PA-C; and Jack Johnson, BS
Executive Summary
This article provides an in-depth clinical review of two major diabetes-related complications: diabetic peripheral neuropathy (DPN) and peripheral arterial disease (PAD). It outlines the etiology, risk factors, pathophysiology, clinical features, diagnostic strategies, and treatment options for both conditions. Emphasis is placed on early detection, prevention, and the importance of comprehensive risk factor management in improving outcomes. The authors offer practical screening tools and therapeutic guidance that are especially relevant for primary care providers managing patients with diabetes or prediabetes.
- Screening and prevention: Annual screening for DPN should begin at type 2 diabetes mellitus diagnosis and five years after type 1 diabetes mellitus diagnosis; patients with prediabetes and metabolic syndrome also should be screened because of increased risk.
- Diagnostic tools: Use pinprick, tuning fork, Semmes-Weinstein monofilament, proprioceptive hallux, and reflex testing to assess sensory function and risk of complications in patients with suspected DPN.
- PAD detection: Ankle-brachial index testing is recommended every five years in patients older than 50 years of age with diabetes or with 10 or more years duration of diabetes; consider toe pressure measurements in cases with calcified, noncompressible vessels.
- Medication management: Exercise caution when prescribing gabapentin because of potential misuse and limited efficacy; consider alternatives such as duloxetine and tricyclic antidepressants for DPN-related pain.
- Foot care: Encourage patient education on daily foot inspections, proper footwear, and prompt referral to podiatry for skin breakdown or ulceration to prevent amputations.
- Risk factor control: Tightly manage glucose, blood pressure, and lipid levels to reduce the progression of both DPN and PAD; smoking cessation and physical activity are critical lifestyle interventions.
- Systemic implications: Recognize that PAD may be asymptomatic and serves as a marker for systemic atherosclerosis, increasing the risk for coronary and cerebrovascular events.
This issue is the second of a two-part discussion of complications of diabetes. Part I discussed the management of chronic kidney disease in people with diabetes. Part II discusses additional microvascular and macrovascular complications of diabetes, including diabetic peripheral neuropathy and peripheral artery disease. We hope these two issues will be useful to your clinical practice.
— Gregory R. Wise, MD, FACP, Editor
Diabetes mellitus (DM) continues to assume pandemic proportions worldwide and is increasing at an alarming rate, which likely will drive an increase in its related micro- and macrovascular complications. This issue of Primary Care Reports will discuss several of these important microvascular and macrovascular complications affecting patients with type 1 and type 2 DM, including diabetic peripheral neuropathy (DPN) and peripheral arterial disease (PAD).
Diabetic Peripheral Neuropathy
Etiology
Given the significant prevalence of diabetes in the population, it is important for the primary care clinician to be familiar with the many types of neuropathies commonly affecting people living with diabetes: peripheral neuropathy, autonomic neuropathy (cardiovascular autonomic neuropathy, genitourinary disturbances [including erectile dysfunction], gastrointestinal neuropathies), proximal neuropathy (diabetic polyradiculopathy), mononeuropathy (focal neuropathy), and Charcot neuroarthropathy. Each type of neuropathy differs by its location, causes, and clinical sequelae. By far the most prevalent type is chronic diabetic peripheral sensorimotor neuropathy, affecting up to 50% of people with diabetes.1 This article aims to interpret the epidemiology, etiology, screening processes, diagnostic measures, and available treatments for DPN only.
DPN is defined as the presence of symptoms and/or signs of peripheral nerve dysfunction in patients with DM, after exclusion of other causes.2 The cause of DPN is multifactorial, but the exact mechanism remains uncertain. DPN is one of the most common chronic complications associated with DM, affecting an estimated 8% to 51% of people with type 2 diabetes (T2DM) and 11% to 50% of people with type 1 diabetes (T1DM).3
Although the primary care clinician’s duty is to have satisfactory knowledge to diagnose and treat DPN in its early stages, preventing its development by effectively managing both metabolic factors and glycemic levels also is of utmost importance.3 Left undiagnosed, DPN can have devastating consequences, since peripheral neuropathies are the leading cause of leg amputations in Western countries.4 Unfortunately, DPN often is diagnosed late when irreversible nerve injury has occurred, and its first presentation may be with a diabetic foot ulcer.
The prevalence of DPN increases with both older age and diabetes duration, although major risk factors vary somewhat depending on diabetes type. Among patients with T1DM, older age and loss of consciousness because of severe hypoglycemic episodes are both positively correlated with the development of DPN. For patients with T2DM, risk factors include older age, adverse cardiometabolic profile, low physical activity, and limited range of motion.5
The need to screen patients who have prediabetes and metabolic syndrome for DPN also should be mentioned. Both conditions are significant risk factors in the development of diabetes, and a growing body of literature links prediabetes, obesity, and metabolic syndrome (a well-known syndrome characterized by metabolic abnormalities, including android obesity, insulin resistance, poor glucose regulation, hypertension, and dyslipidemia) to an increased risk of developing peripheral neuropathy.6 The literature shows that patients with prediabetes can have DPN-like symptoms, such as sensory neuropathy and/or neuropathic pain. Since prevention and early detection of peripheral neuropathy are paramount, the importance of screening all patients with metabolic syndrome and prediabetes cannot be overstated.7 (For further information, see Table 1 here: https://www.aafp.org/pubs/afp/issues/2020/1215/p732.html)
Epidemiology
According to the 2021 report by the International Diabetes Foundation (IDF), approximately 537 million individuals globally were affected with diabetes, and projections indicate a surge in diabetes cases to 783 million by 2045.8 Thus, it is safe to assume that the number of patients with DPN also will increase. The incidence of DPN is higher in people with T2DM than in those with T1DM, although the prevalence is similar in both groups: 8% to 51% in T2DM compared to 11% to 50% in T1DM.3
DPN is a major cause of diabetes-associated morbidity and mortality. The morbidity associated with DPN can be devastating, and includes development of foot ulcers, amputations, falls, intracranial injuries, pain, depression, and poor sleep. One or more of these factors often leads to a significantly impaired quality of life for the patient with DPN. Research also has shown that peripheral neuropathy is common and is independently associated with mortality in the U.S. population, even in the absence of diabetes, which again highlights the need to screen all patients, especially those with prediabetes and metabolic syndrome.9
Pathophysiology
DPN encompasses several pathologies that manifest from complications in the peripheral nervous system. This phenomenon is characterized by segmental demyelination and axonal degradation in both the somatic and autonomic divisions of the peripheral nervous system.10 The origins of such complications are multifactorial, and the exact cause of DPN remains uncertain.
The pathophysiologic mechanisms that cause neuropathic damage are thought to involve both metabolic and intracellular processes; however, these mechanisms still are not fully understood. Glucose variability and hyperglycemia appear to promote ischemic nerve damage through several systemic inflammatory processes. Microvessels found in peripheral nerves are lined by a blood-nerve barrier within the endoneurium and commonly are disrupted in the presence of chronically hyperglycemic patients. One proposed mechanism is an inflammatory cascade of events that increases the permeability of this barrier. This leads to hyperpermeability of heavy proteins into the endoneurium and, by extension, promotes the progressive deposition of edema through the thickening of the external laminae.4
Damage to nerve cells from the oxidative stress created by reactive oxygen species (ROS) in people with hyperglycemia also has been implicated.10 The distortion in the redox balance because of the overproduction of ROS and the dysregulation of antioxidant defense systems promotes the oxidative damage in DPN.11
Clinical Features
DPN is a diagnosis of exclusion. A patient with diabetes may present with peripheral neuropathy of nondiabetic origin, which may be treatable.2 Therefore, causes of neuropathy other than diabetes should be considered, including toxins, neurotoxic medications (such as chemotherapy), hypothyroidism, renal disease, malignancies, infections, chronic inflammatory demyelinating neuropathy, inherited neuropathies, vasculitis, and vitamin B12 deficiency.12 Particularly relevant in patients with T2DM who are taking metformin for glycemic control, metformin-induced vitamin B12 deficiency should be considered.13
Metformin is the ninth most often prescribed medication in the world. It is estimated that 120 million patients with diabetes receive the drug.14 Because of the widespread use of metformin in the management of T2DM, increasing evidence over the past two decades suggests that patients who are treated with metformin can develop vitamin B12 deficiency.14-16 Metformin has been association with interference in vitamin B12 absorption and deficiency; thus, screening for vitamin B12 deficiency in appropriate patients should not be overlooked.17
Although it is not recommended to routinely screen all patients taking metformin, it is prudent to abide by the current recommendations made in the 2023 American Diabetes Association Standards of Care, which state, “Long-term use of metformin may be associated with biochemical B12 deficiency; consider periodic measurements of B12 in metformin-treated individuals, especially those with anemia or peripheral neuropathy.”18
The most common presentations of DPN include, but are not limited to, numbness, tingling, aching, burning sensations, and weakness of limbs.19 Early DPN symptoms most commonly involve small fiber disturbances, presenting as dysesthesias (unpleasant sensations of burning and tingling).2 Later in the natural progression of DPN, disturbances in large fibers that regulate the proprioceptive signals of vibration, touch, and balance occur.
This large fiber damage has significant clinical importance, since it has the potential to cause numbness and a phenomenon known as loss of protective sensation (LOPS).2 Patients with LOPS have varying degrees of insensate extremities and may not have any complaints of injury or pain in affected extremities because of physical unawareness. The absence of this most basic nociceptive mechanism (lack of ability to sense tissue damage) results in the patient’s inability to perceive local foot trauma both from intrinsic factors, such as abnormal or faulty foot mechanics or deformity (Charcot’s neuroarthropathy), as well as from extrinsic factors such as foreign objects or improper footwear. This places the foot at increased risk for developing an ulcer, which ultimately can lead to amputation.20 Additionally, as a result of the effect of DPN on balance, patients with large fiber disturbance have a five-fold increased risk of falling, and the consequences include injuries, decline in mobility, activity avoidance, institutionalization, and mortality.4,21
As discussed earlier, the clinical features or symptoms of DPN vary according to the class of sensory fibers involved. Early symptoms most commonly are caused by the involvement of small fibers: pain and dysesthesias (burning and tingling). With the onset of large fiber involvement, numbness and LOPS occur. The pattern of sensory symptoms in DPN patients occurs in a stocking-glove pattern — the most distal portions of peripheral nerves are affected first, then symptoms move proximally.
DPN, similar to diabetic retinopathy, should be assessed at the time of diagnosis of T2DM and five years after the diagnosis of T1DM. Following this initial assessment, patients with T1DM and T2DM should be screened annually for DPN.2
The following clinical tests may be used to assess small-fiber function, large-fiber function, and protective sensation (see Figure 1):2
- Small-fiber function: pinprick and temperature sensation;
- Large-fiber function: vibration perception, 10-g monofilament, reflexes and proprioception;
- Protective sensation: 10-g monofilament.
Figure 1. Instruments Used in Screening for Neuropathy |
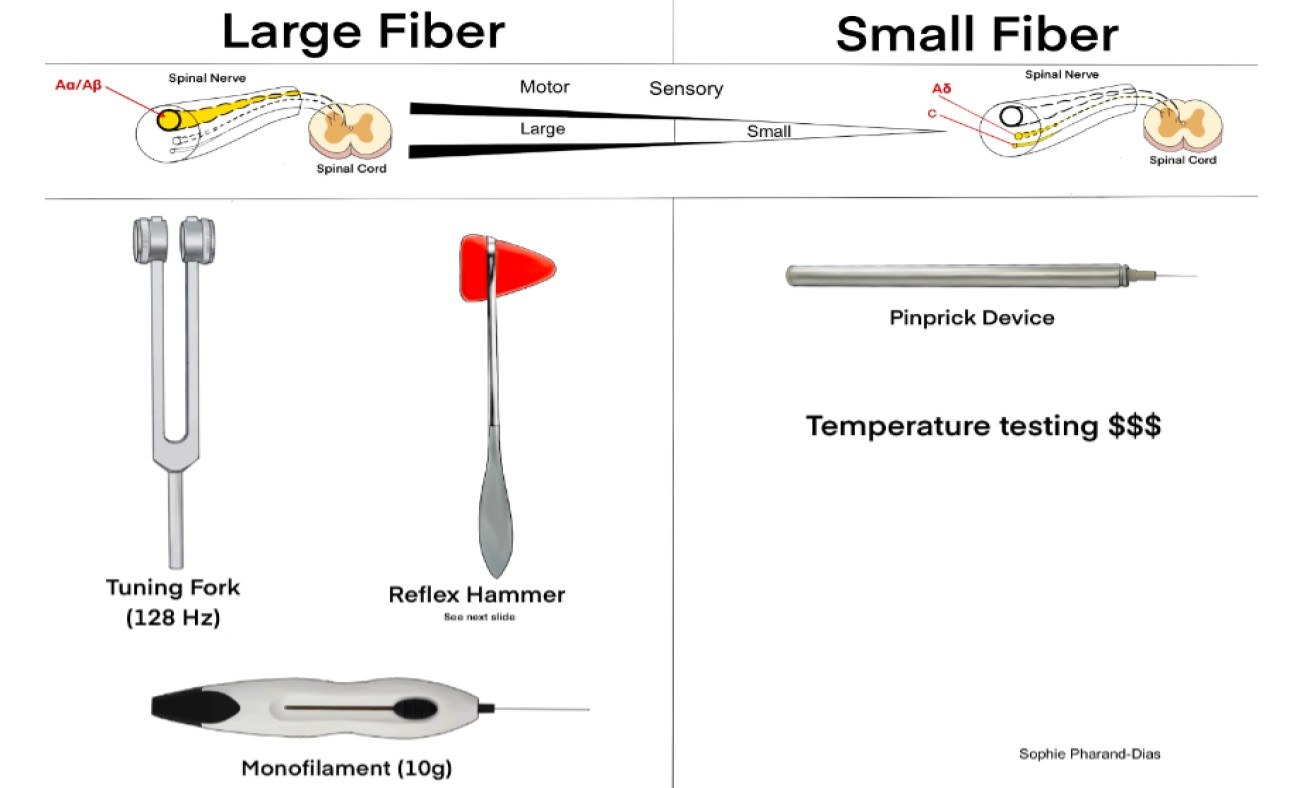 |
Illustration by Sophie Pharand-Dias |
These tests not only screen for the presence of dysfunction but also predict future risk of complications. Electrophysiological testing or referral to a neurologist rarely is needed, except in situations where the clinical features are atypical or the diagnosis is unclear.12
The patient should be prepared for the visit with socks and shoes removed. If available, a well-trained and verifiably competent medical assistant can perform and document pinprick, monofilament, and tuning fork testing prior to the primary care clinician seeing the patient. Additionally, education regarding proper footwear, daily foot checks, and checking the shoes for foreign objects prior to donning them can be provided by the medical assistant. Documentation of any abnormal physical findings should be made at each encounter and are critical to avoid DPN complications. If there are any skin changes or skin breakdown, it should be emphasized that an emergent referral to podiatry is warranted. Furthermore, patients should be strongly encouraged to wear closed-toe shoes at all times.
Clinical Testing: Small Fiber Function: Pinprick Testing
Pinprick testing in patients with DPN has proven to be a reliable measure of testing small-fiber function. The pinprick threshold (PPT) is the lightest weight needle that consistently elicits a sharp sensation.22 There is no clear consensus on the best pinprick needle size; however, the needle size is not as important as the amount of pressure applied when using the needle. Neurotips and Medipin, which both weigh ~40 g, commonly are used in clinical settings. To apply the proper pressure, gently place the needle on the skin so that the needle’s weight is the primary force. Patients should be instructed to report pinprick (sharp) sensation only, not tactile sensation.22 Two commonly tested sites are the anatomical snuff box (space between the first two metacarpals) and the dorsal side of both feet on the proximal portion of the first intermetatarsal space.22
Temperature Testing
In clinical practice, this testing is not routinely done because of the lack of cost-effective options; however, temperature sense can be perfunctorily tested with a cold tuning fork.23
Clinical Testing: Large-Fiber Function: Tuning Fork Evaluation
The 128-Hz (non-graduated) tuning fork is a simple and traditional way to evaluate large-fiber function via vibratory sensation.2,24 (See Figure 2.) An abnormal response is when a patient fails to perceive the vibratory sensation. Two methods exist: the on-off method and the timing method. The on-off method involves striking the tuning fork, initiating vibration, then applying the base of the tuning fork to the bony prominence on the dorsum of each hallux while the patient’s eyes are closed. The patient should be asked if the vibration is sensed (“on”) and when the vibration has stopped (“off”).24 The timing method involves applying the tuning fork to the same bony prominence of each hallux. The patient should be asked to report the time at which vibratory sensation has diminished beyond perception.25
Figure 2. Testing Vibratory Sensation with 128-Hz Tuning Fork |
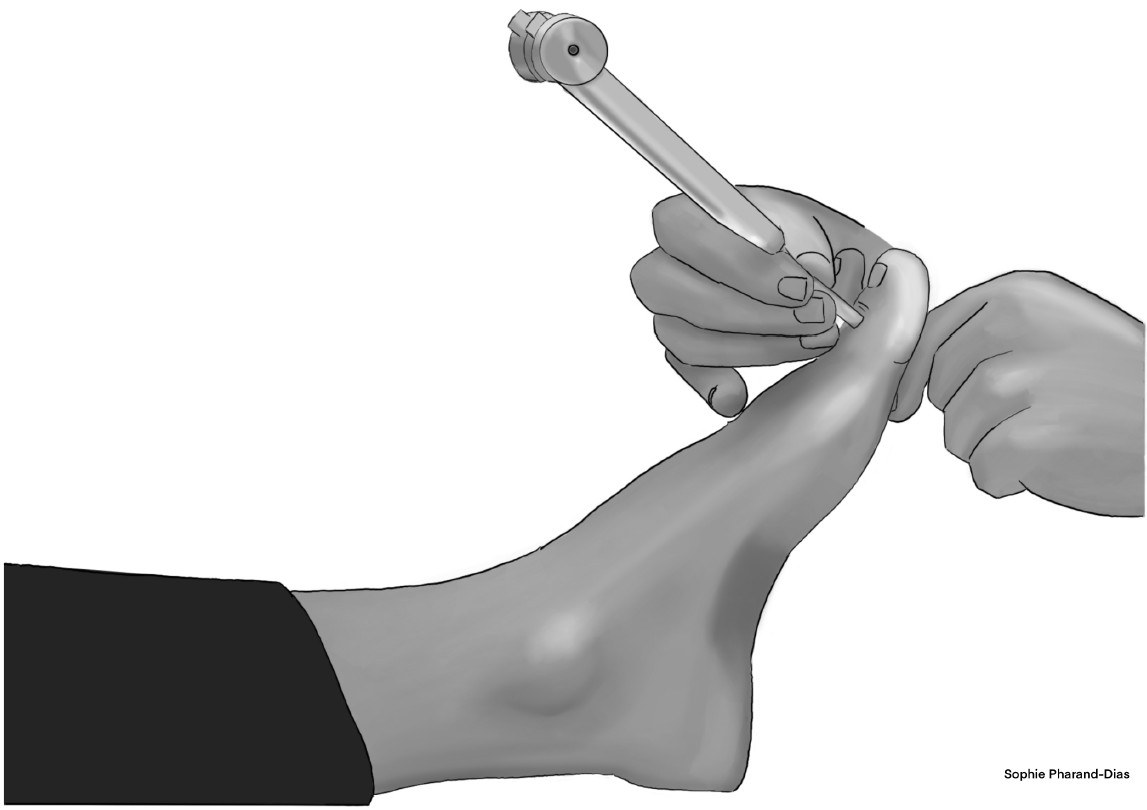 |
Illustration by Sophie Pharand-Dias |
Semmes-Weinstein Monofilament Sensory Test
The Semmes-Weinstein monofilament sensory test (SWMT) involves a 10-g monofilament, which can be used to evaluate large-fiber function as well as protective sensation. (See Figure 3.) The recommended sites for SWMT include the first, third, and fifth metatarsal heads and the plantar surface of the distal hallux.25 Other sites also can be tested as shown in Figure 3. While the practitioner applies appropriate force (the appropriate force is when the monofilament begins to bend), the patient (with eyes closed) should be asked to answer yes or no to indicate the sensation of the monofilament and to describe the correct location of the monofilament sensation. If a patient responds no, it suggests the presence of anaphia (tactile anesthesia) at the respective site.25
Figure 3. Semmes-Weinstein Monofilament Sensory Test |
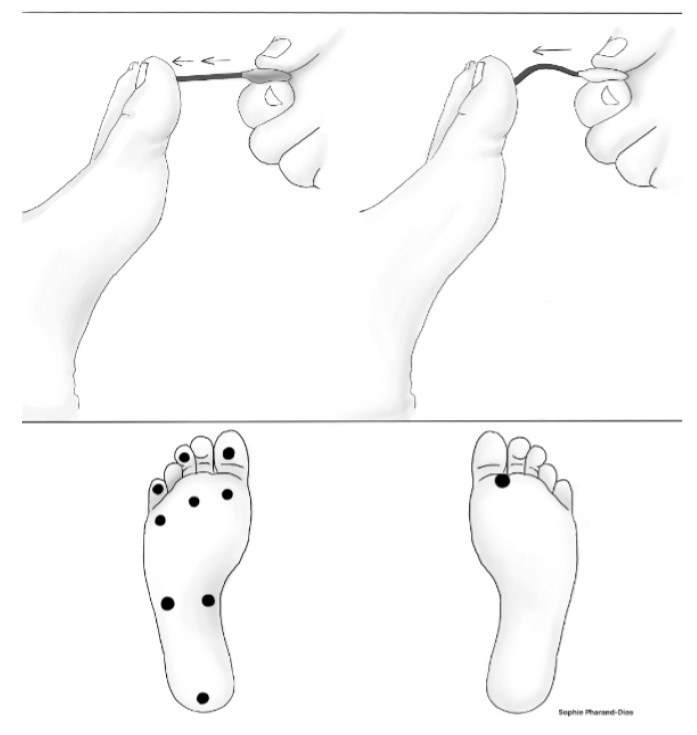 |
Illustration by Sophie Pharand-Dias |
Proprioceptive Hallux Test
A lesser known but nonetheless effective sensory lower extremity test that also tests for large-fiber dysfunction is the proprioceptive hallux test. (See Figure 4.) Moving the hallux (big toe) up, down, and side to side while the patient keeps their eyes closed is a cost-effective and efficient way for evaluating lower extremity deficits. If a deficit is observed in the proprioceptive hallux test, then testing of foot (ankle joint) proprioception should be performed next to determine the extent of the deficit.
Figure 4. Proprioceptive Hallux Test |
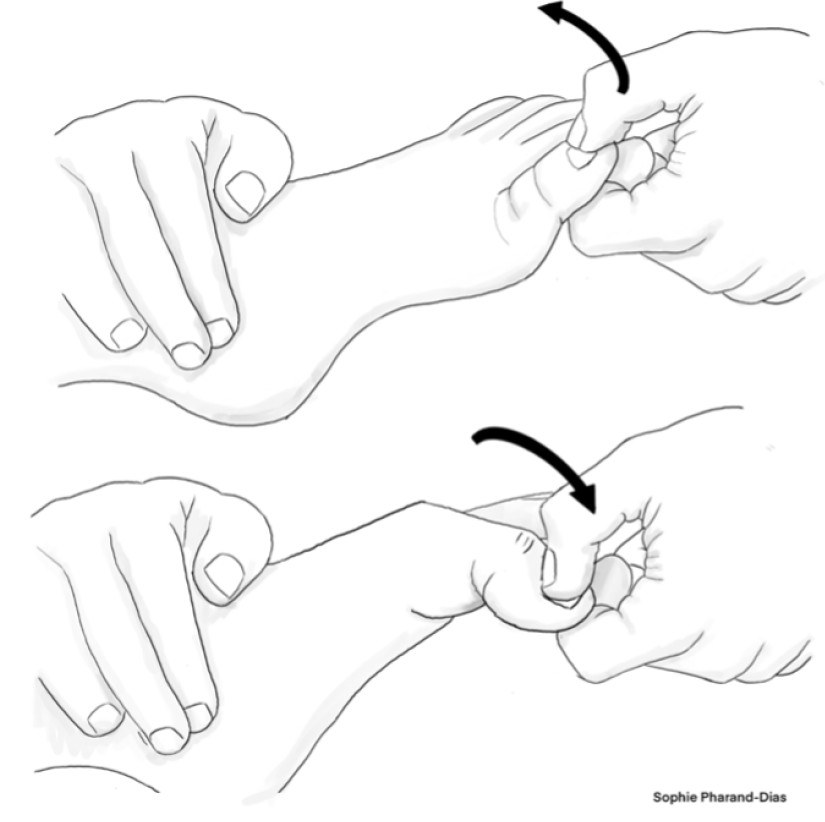 |
Illustration by Sophie Pharand-Dias |
Reflex Testing
Decreased or absent reflexes often indicate mild and/or asymptomatic DPN. As such, the patellar and Achilles tendon reflexes are common testing methods that can reflect the degree of injury in peripheral nerves. In the absence of reflex, the test can be repeated with reinforcement. Although several scales exist, reflexes commonly are graded as: 0 = absent, 1 = somewhat diminished/requires reinforcement (hypoactive), 2 = normal, 3 = brisker than average (hyperactive), or 4 = very brisk (with clonus). (See Figures 5a and 5b.)
Figure 5a. Patellar Reflex |
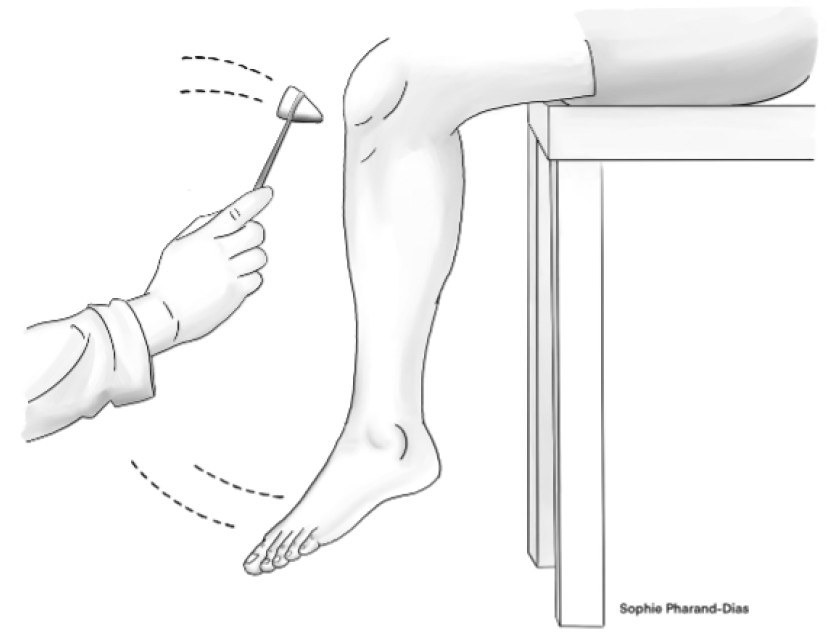 |
Illustration by Sophie Pharand-Dias |
Figure 5b. Achilles Reflex |
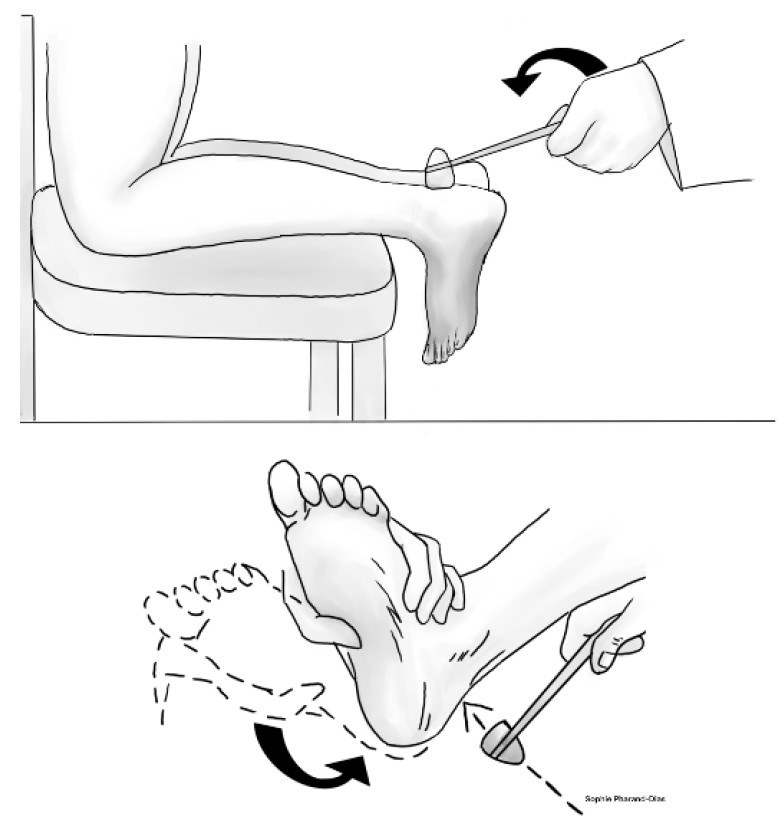 |
Illustration by Sophie Pharand-Dias |
Treatment/Management
Given that glucose dysregulation plays a major role in the development of DPN, optimizing glucose levels is the first step in prevention. Helping patients to consistently achieve optimal glucose levels can prevent and delay neuropathy in T1DM and can slow the progression of neuropathy in T2DM.2
Traditionally, the management of diabetes has been glucocentric, meaning its management has focused mainly on the optimization of blood glucose levels; however, during recent years, an accumulation of evidence has highlighted the importance of treating T2DM in a more holistic, cardiorenal-metabolic approach and, thus, the next order of business is to address the patient’s metabolic risk factors by optimizing the patient’s blood pressure and lipid control.26 Proper blood pressure and lipid management can reduce the risk of developing DPN and slow its progression. Exhibiting caution when prescribing blood pressure medications is urged for patients with cardiovascular autonomic neuropathy (CAN) because of the increased risk of orthostatic hypotension.
Assessing pain levels and treating pain is paramount in improving quality of life after a patient has been diagnosed with DPN. Referral to a neurologist or pain specialist does not become necessary until the treating physician cannot achieve adequate pain control.2
When treating neuropathic pain in patients with DPN, the first class of medications that often comes to mind is gabapentinoid drugs: gabapentin and pregabalin. Gabapentin was patented in 1977 and approved by the U.S. Food and Drug Administration (FDA) in 1993 as an antiepileptic drug.27 The transition of gabapentinoids into a first-line pain medication is in part the result of an intentional marketing strategy by the pharmaceutical industry (now well-documented in the medical literature) that involved widely promoting off-label use with low-quality, industry-funded studies manipulated to exaggerate the perceived analgesic effects of these drugs. The idea that these drugs are highly effective first-line options for any pain defined as neuropathic simply is incorrect.28
Gabapentin, also referred to by its street name, johnnies, initially was marketed as a medication with no significant risk for abuse; however, there has been increasing evidence of the potential for gabapentin misuse and abuse. It is known that gabapentin at higher doses, especially when combined with central nervous system (CNS) depressants, can cause respiratory failure, as can be seen in people who abuse opiates.29 This danger is recognized, and has resulted in changes in state laws. Gabapentin is not a controlled substance according to the federal government, but as of July 2022, seven states (Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia) have passed their own laws classifying gabapentin as a Schedule V controlled substance, and 12 other states currently have mandatory gabapentin reporting requirements.30
Although there is a percentage of patients who have subjective pain relief at higher doses of gabapentin (1,800 mg to 3,600 mg in daily divided doses), considering that more than half of patients treated with gabapentin will not have worthwhile pain relief and that titrating to these doses can be limited by side effects, use of pharmacological agents other than gabapentinoids becomes important, especially in vulnerable populations (for example, older patients or patients with chronic obstructive pulmonary disease and/or obstructive sleep apnea).21
Other first-line pharmacotherapeutic agents for painful DPN that can be used concomitantly in patients who have some but not complete relief with gabapentinoids or can be used on their own include the selective serotonin noradrenaline reuptake inhibitors (SNRIs). SNRIs increase the synaptic availability of 5-hydroxytryptamine and noradrenaline, increasing the activity of descending pain inhibition pathways.31 Duloxetine is used widely and was found in a recent systematic meta-analysis of randomized controlled trials to be more efficacious than placebo treatments in patients with painful DPN. When a 60-mg dose is insufficient, 120 mg of duloxetine may improve DPN symptoms; severe adverse events have been reported to be rare.32
Tricyclic antidepressants have multimodal analgesic action, including blocking serotonin and noradrenaline reuptake from synaptic clefts and varying degrees of anticholinergic receptor inhibition.33 Despite the anticholinergic side effect profile, which limits its use in patients who are older or who have cardiac disease, amitriptyline remains a viable treatment option for some patients with painful DPN.
There are other treatments that have been studied and prescribed for painful DPN with inconclusive evidence for benefit. They include narcotics (whose side effects limit their utility), lidocaine and capsaicin patches and creams, vitamin D supplementation for patients who are deficient, and acupuncture and other alternative therapeutic modalities.33
Of note, many recent studies have looked for evidence that some antidiabetic drugs may not only have a significant role in controlling the hyperglycemia caused by diabetes, but may prevent some of its complications, including DPN. In one such study, it was shown that sodium-glucose co-transporter 2 inhibitors (SGLT2 inhibitors) moderately improved the manifestations of diabetic peripheral neuropathy events and nerve conduction velocity.34
Studies of the glucagon-like peptide-1 receptor agonists (GLP-1R agonists) have shown that this class of medications acts on the nervous system, providing neuroprotective effects. The required cardiovascular safety randomized controlled trials on the GLP-1R agonists have furthered understanding of their beneficial effects on patients with high vascular risk. Emerging evidence suggests that their use could be an interesting treatment strategy to reduce neurological complications, including DPN. A recent systematic review examining this evidence revealed that although strong studies exist regarding the effects of GLP-1R agonists on stroke and major adverse cardiovascular events (MACE), more information is required to extrapolate these results to other neurological fields, since they seem to be very poor and insufficient in the fields of cognitive impairment and peripheral neuropathy.35
Additional Aspects: Cost
DPN is a major complication of diabetes requiring costly medical treatment. The total annual cost of drug therapies is higher in DPN patients compared to non-DPN patients. In 2003, it was estimated that the total annual cost of DPN was between $4.6 and $13.7 billion.36 No study has evaluated the economic burden of DPN in recent years, but the 2003 annual cost translates to between $8.5 and $25.5 billion today.37
Summary
Currently, DPN affects up to half of patients living with diabetes. Peripheral neuropathy also can be present in patients with prediabetes and/or metabolic syndrome.
Management of glucose and metabolic risk factors is the most important tools the clinician has to prevent the development of peripheral neuropathy. Screening, early detection, and patient education remain critical.
DPN is a diagnosis of exclusion. While the cornerstone of treatment is prevention, after the diagnosis of DPN is made, aggressive use of available treatments to improve the patient’s quality of life and to decrease morbidity and mortality are of utmost importance.
Peripheral Arterial Disease in Diabetes
Peripheral arterial disease (PAD) refers to partial or complete occlusion of peripheral vessels of the lower extremities. The underdiagnosis of PAD in the primary care setting may be a major issue because of many patients presenting without the typical claudication symptoms described in medical textbooks. The ability of primary care clinicians to diagnose PAD in asymptomatic patients still has a significant clinical effect because PAD acts as a marker for systemic atherosclerosis.38
Etiology
Unfortunately, even though PAD is associated with, and is an independent predictor of, both cardiovascular (CV) and cerebrovascular ischemic events, it largely is underdiagnosed and undertreated in people with diabetes. Patients with PAD have decreased lower extremity arterial perfusion, which often is described as poor circulation. Typically, in PAD, atherosclerotic plaques narrow the arterial lumen, which restricts blood flow to the distal extremity. Atherosclerosis is a progressive process affecting multiple vascular beds. Its clinical consequences, which include coronary artery disease (CAD) and cerebrovascular disease in addition to PAD, potentially are life-threatening.39 Atherosclerotic disease in one vascular bed indicates possible disease in others.40
Approximately 150,000 patients undergo a lower extremity non-traumatic amputation in the United States annually.41 As the incidence and prevalence of diabetes increases annually worldwide, the number of amputations also rises.42 Most commonly, these amputations are the result of DM, peripheral vascular disease, neuropathy, soft tissue sepsis, and trauma. Early identification of risk in a non-healing diabetic foot ulcer can avoid an amputation of a lower extremity that can be devastating, debilitating, and demoralizing.43
Epidemiology
There are confounding variables that limit the determination of the true prevalence of PAD in patients with diabetes. To be sure, the prevalence of PAD depends on the method of diagnosis; however, irrespective of the method of diagnosis, prevalence typically increases with advancing age. Another confounding variable is that many people with diabetes and PAD are asymptomatic, and have peripheral neuropathy (which may alter pain perception), absent peripheral pulses (dorsalis pedis and posterior tibial), and claudication.
A recent study investigating the prevalence of CAD, stroke, and PAD rates in older adults found that 3% of the population (n = 1,802) had PAD alone.40 Among older adults with PAD, it was observed that 68% had coexisting CAD.40 As mentioned previously, the presence of atherosclerotic disease in one vascular bed indicates possible disease in other vascular beds.
Risk Factors
The three most common risk factors for PAD include smoking, diabetes, and increasing age. These are followed by dyslipidemia, hypertension, male gender, and race. Although PAD traditionally has been seen as a disease affecting men, the prevalence of PAD appears to be equal among senior men and women. It has been observed in multiple long-range studies that the rule in PAD is that the patient is asymptomatic.
There is a strong positive association between the duration of DM and the risk of developing PAD, especially among men with hypertension or who are current smokers. The degree of diabetic control is an independent risk factor for PAD. In fact, with every 1% increase in A1c, the risk of PAD has been shown to increase by 28%, and people with diabetes are 15 times more likely to have an amputation than those without.44,45 With respect to gender distribution, it has been shown that the rate of intermittent claudication (IC) in men was double that in women in all age groups.46
The Clinical Effect of PAD
The mortality rate in patients who have PAD is two to three times higher than in age- and sex-matched controls.47 The five-year mortality rate is 15% to 30% after PAD is diagnosed, and the 15-year mortality rate is 70% after PAD is diagnosed.48 Approximately 60% to 80% of patients with PAD have CAD in at least one vessel and approximately 14% of PAD patients have an asymptomatic carotid artery stenosis of greater than 70%.49,50 The clinician can screen for carotid stenosis on a physical exam by using the stethoscope to listen for bruits (abnormal whooshing sounds caused by the turbulent blood flow of a narrowed artery). The presence of a bruit may warrant a carotid ultrasound and, potentially, an angiogram.
Differential Diagnosis
When evaluating a patient for PAD, the clinician must distinguish between PAD and other conditions with similar presentations. There are some differentiating symptoms that should be discussed with patients that one might suspect of having IC. A key point is that IC might involve a cramping pain that occurs when walking a certain distance (one block, two blocks, etc.) that does not resolve with continued activity. It might occur in one or both legs and affect the foot and/or calf moving upward/proximally. It abates with rest (while standing) or while reducing the walking speed. This is unlike pseudoclaudication or spinal stenosis, where patients may experience a sharp/paresthetic pain with or without numbness. It varies with walking distance; however, patients may obtain relief by sitting or leaning forward. The discomfort/pain may start at the lower back or thigh level and move downward. There are rarer causes of symptoms with fibromuscular dysplasia (FMD), iliac syndrome in cyclists, Buerger’s disease, and large vessel vasculitis (i.e., Takayasu’s and giant cell arteritis).
Clinical Exam and Features
An annual comprehensive foot evaluation is important to identify risk factors for ulcers and amputations.2 The exam should include an inspection of the skin, an assessment for foot deformities, a neurological assessment (as discussed previously in the section on DPN), and a vascular assessment of the pulses of the legs and feet. If a patient has had any prior ulcerations or amputations, their feet should be inspected at every visit.
When evaluating the patient, any prior history of the following should be noted: lower extremity ulcerations or amputations, Charcot foot, angioplasty or vascular surgery, nicotine use (cigarettes, chewing tobacco, nicotine pouches or ZYN, and vaping), retinopathy, renal disease, erectile dysfunction (ED), or CAD. The clinician also should assess current symptoms of neuropathy (pain, burning, numbness) and vascular disease (leg fatigue, cramping, claudication).
Initial screening for PAD should include an assessment of lower-extremity pulses (see Figure 6), capillary refill time, rubor on dependency, pallor on elevation, decreased or absent hair growth on the lower leg areas, thin skin, and venous filling time. Individuals with a history of leg fatigue, claudication, decreased or absent pedal pulses, and rest pain relieved with dependency should be referred for ankle-brachial index (ABI) with toe pressures and for further vascular assessment as appropriate.
Figure 6. Location of the Posterior Tibial Artery and the Dorsalis Pedis Artery |
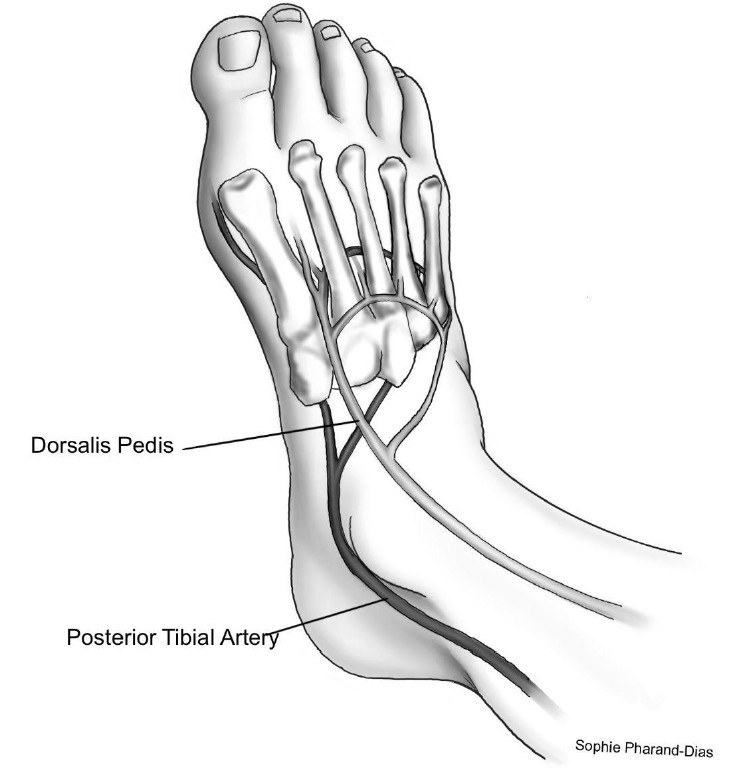 |
Illustration by Sophie Pharand-Dias |
A Diabetic Foot Examination form can be downloaded from the American College of Physicians website and may be helpful to the clinician: https://www.acponline.org/sites/default/files/documents/running_practice/practice_management/forms/dm-diabetic-foot-exam.pdf
ABI Screening
The ABI is defined as the ratio of the ankle systolic blood pressure divided by the brachial systolic blood pressure. Normal is between 1.00 and 1.40.51 In PAD, the ankle systolic pressure is less than the brachial systolic pressure, and the ABI is reduced to < 1.00; PAD is defined as an ABI < 0.90.
The American Diabetes Association (ADA) and the American Heart Association recommend ABI testing for people with diabetes who are older than 50 years of age or who have had DM for at least 10 years. The test should be repeated every five years.2 An ideal ABI is 0.9 to 1.3. Be aware that ABI determinations may be of limited value in some patients with diabetes because calcification of the tibial arteries may render them noncompressible, resulting in unusually high ABI values (> 1.40).52
Ankle-brachial indices are to be carefully evaluated because of the fact that there is the possibility of inaccuracy in people with diabetes secondary to noncompressible vessels. Toe systolic blood pressure tends to be more accurate in this population. These brachial systolic readings of < 30 mmHg are suggestive of PAD and an inability to heal foot ulcerations. These patients should be referred for immediate vascular evaluation. More advanced and invasive imaging, such as angiography, may be needed.
Further Testing for PAD
Any individual exhibiting signs and symptoms of PAD should be referred for noninvasive arterial studies in the form of Doppler ultrasound with pulse volume recordings. Duplex ultrasound is indicated as the first-line imaging technique to detect the site and extent of vascular lesions.
Treatment
Risk Factor Modification
Atherosclerotic risk factors for PAD include cigarette smoking, diabetes, dyslipidemia, and hypertension. Smoking cessation increases long-term survival in patients with PAD. Most of the risk reduction in any diabetes-related aggregate endpoint was the result of a 25% reduction in microvascular endpoints rather than macrovascular endpoints. There are no direct data on treating dyslipidemia in patients with diabetes and PAD. However, because of the risk of having multiple vascular beds (cardiac and cerebrovascular) affected, low-density lipoprotein cholesterol should be less than 70 mg/dL in these patients. Regarding treatment of hypertension, the role of intensive blood pressure control in patients with diabetes and PAD has not been established; however, using the ADA Standards of Care, a blood pressure goal of ≤ 130/80 mmHg is warranted in all patients with diabetes.2
Additional lifestyle changes, such as tighter control of blood sugar and engaging in supervised exercise programs, should be considered, since better blood sugar control can be helpful for a multitude of reasons. It is known that high blood sugar levels cause proteins and lipids in the blood vessels to bond abnormally, forming advanced glycation end products (AGEs). These AGEs can make blood vessels stiffer and less responsive, contributing to reduced blood flow and increased plaque formation. In addition, poor circulation and hyperglycemia can impair the immune response, slowing the healing of wounds. When the blood sugar is out of control, these mechanisms can contribute to the development of chronic foot ulcers, which may become infected, possibly resulting in amputation.
Interventions
GLP-1R agonists may have beneficial effects in the context of CV and PAD health. Some proposed mechanisms include improved endothelial function, decreased inflammation, reduction in atherosclerosis, improvement of glycemic control, weight loss, lowering blood pressure, and the possibility of direct CV benefits. Currently, the most prominent trial investigating GLP-1R agonists for PAD is the STRIDE Trial. If positive results are found, this could establish a new treatment option (semaglutide 1.0 mg once per week subcutaneously) for managing PAD symptoms with GLP-1R agonists.
Regarding the use of other antidiabetic agents in patients with PAD, it should be mentioned that a placebo-controlled retrospective randomized trial recently published in Diabetes Care found that among older veterans with T2DM, there was an increased risk for amputation with the use of SGLT2i in patients with underlying CV disease; however, the evidence remains unconfirmed by other trials. It should be noted that the primary outcome of this trial was the time to the first surgical event for PAD (amputation, peripheral revascularization and bypass, or peripheral vascular event). A hazards model was used to compare the event risk between the two study groups: those taking SGLT2 inhibitors and those taking dipeptidyl peptidase-4 (DPP-4) inhibitors. The cumulative probability of PAD-related surgical events at four years was higher for SGLT2 inhibitor users than for DPP-4 inhibitor users (4.0% vs. 2.8%, respectively). The authors stated that results underscore the need to determine the safety of SGLT2 inhibitor use among patients with diabetes who remain at very high risk for PAD.53
Antiplatelet therapy and aspirin have several indications in the treatment of PAD. These agents should be used in patients who may have an endovascular shunt placed in an attempt to improve circulation in an obstructive area. Additionally, patients with symptomatic PAD should be initiated on antiplatelet therapy (aspirin 75 mg to 325 mg daily or clopidogrel 75 mg daily).54 In more severe cases of PAD, procedures such as angioplasty, stenting, or surgical bypass may be needed to restore blood flow or avoid amputation.
An interprofessional team approach is recommended for individuals with foot ulcers and high-risk feet (e.g., dialysis patients, Charcot foot, history of foot ulcers and/or amputations, and PAD). Also, refer patients who use nicotine products and with a history of prior lower-extremity complications, LOPS, structural abnormalities, or PAD to footcare specialists.
Specialized therapeutic footwear is recommended for patients with a history of ulcers, loss of sensation, foot deformities, callous formations, a history of amputations, or poor peripheral circulation. After a patient has LOPS, they should never walk barefoot, even around the house. Shoe fitting is critical and there should be at least one-half inch between the longest toe and the end of the shoe. They should avoid open-toe shoes at all times. Patients with prior issues/problems should be educated regarding how to self-examine feet through palpation or visual inspection with an unbreakable mirror for daily surveillance of early foot problems. Teach patients to check inside their shoes for foreign objects (nails, tacks, other small objects) before donning their shoes, since there is the possibility of objects being lodged through the soles or inside the shoe and potentially causing injury.
For chronic diabetic foot ulcers that have failed to heal with optimal standard care alone, adjunctive treatment with agents proven to be effective in randomized controlled trials should be considered. (See Tables 2 and 3.)
Table 2. Timeline for the Management of Factors Associated with At-Risk Feet | |
Associated Factors | Exam Frequency |
Poor glycemic management | As indicated |
Peripheral neuropathy/LOPS | Every six to 12 months |
PAD | Every six to 12 months |
Foot deformities (bunions, hammertoes, Charcot joint, etc.) | Every three to six months |
Pre-ulcerative corns or calluses | As indicated |
Prior ulcerations | Every one to three months |
Prior amputation | Every one to three months |
Use of nicotine products (smoking, vaping, or chewing) | At every visit |
Retinopathy | As indicated |
Nephropathy (ESRD patients and/or post-transplant) | Every one to three months |
LOPS: loss of protective sensation; PAD: peripheral artery disease; ESRD: end-stage renal disease The information in this table was merged and organized by the authors. | |
Table 3. Categories of Advanced Wound Therapies (Interventions Used When Standard Wound Care Has Failed) |
|
The information in this table was merged and organized by the authors. |
Additional Aspects
Clinical Significance of PAD
The effect on quality of life in patients with PAD is more severe than that of the effect of congestive heart failure (CHF) or recent myocardial infarction (MI). In fact, the effect of critical limb ischemia (CLI) is comparable to terminal cancer.55 Functional impairment and decline is common, even in patients with PAD who are asymptomatic.56 The natural history of CLI one year after diagnosis is: alive with two limbs, 50%; amputation, 25%; and cardiovascular death, 25%.57
Shared Connection Between PAD and ED
ED sometimes can serve as an early warning sign for PAD or other CV diseases. Diabetes not only increases the risk of PAD but also directly contributes to ED through mechanisms such as blood vessel damage, neuropathy, and reduced nitric oxide availability. Nitric oxide is essential for blood vessel relaxation and adequate blood flow to the penis. Endothelial dysfunction impairs blood flow regulation and contributes to the development of atherosclerosis, affecting blood supply to the penis and extremities.
Shared Connection Between PAD and CAD
Having PAD can be viewed as a marker for systemic atherosclerosis and CV disease, requiring comprehensive management to reduce the risk of major CV events. People with PAD are more likely to experience angina, MI, stroke, and other CV events as the result of systemic atherosclerosis. This should be discussed with the patient, since these additional risks are another reason for lifestyle changes (such as a heart-healthy diet, exercise, smoking cessation, and managing blood pressure, cholesterol, and diabetes) to reduce the progression of underlying atherosclerosis.
Summary
Although the exact prevalence of PAD in patients with diabetes is difficult to elucidate, the longer a patient has been living with diabetes and the less well controlled their diabetes is, the greater their risk of developing PAD.
It is essential for the primary care clinician to understand the importance of guideline-directed screening because prevention and early detection of PAD is essential to preserve the patient’s quality of life and to prevent future morbidity and mortality. Lifestyle changes (such as smoking cessation and exercise) and risk factor modification (such as tighter control of blood sugar and adequate management of metabolic risk factors) are helpful in delaying the development of PAD. After PAD develops, treatment currently includes antiplatelet therapy and, in advanced cases, angioplasty, stenting, or surgical bypass may be needed to restore blood flow or avoid amputation.
An interprofessional team approach is recommended for patients with at-risk feet to prevent the development of skin breakdown, ulcers, infection, and necrosis. These complications can be devastating and ultimately may lead to amputations.
Frank Lavernia, MD, is a Volunteer Diabetologist at Caridad Center, Boynton Beach, FL.
Carrie O’Day, PA-C, is a Volunteer Diabetologist at Caridad Center, Boynton Beach, FL.
Jack Johnson, BS, is a student at Florida Atlantic University.
The authors would like to thank Karla J. Holt, BS, and Sophie Pharand-Dias, BS, for their graphics and illustrations contributions to this manuscript.
References
1. Jende JME, Groener JB, Oikonomou D, et al. Diabetic neuropathy differs between type 1 and type 2 diabetes: Insights from magnetic resonance neurography. Ann Neurol. 2018;83(4):588-598.
2. American Diabetes Association Professional Practice Committee. 12. Retinopathy, neuropathy, and foot care: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(Suppl 1):S231-S243.
3. Gandhi M, Fargo E, Prasad-Reddy L, et al. Diabetes: How to manage diabetic peripheral neuropathy. Drugs Context. 2022;11:2021-10-2.
4. Galiero R, Caturano A, Vetrano E, et al. Peripheral neuropathy in diabetes mellitus: Pathogenetic mechanisms and diagnostic options. Int J Mol Sci. 2023;24(4):3554.
5. Pfannkuche A, Alhajjar A, Ming A, et al. Prevalence and risk factors of diabetic peripheral neuropathy in a diabetics cohort: Register initiative “diabetes and nerves.” Endocr Metab Sci. 2020;1(1-2):100053.
6. Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome.J Diabetes Investig. 2017;8(5):646-655.
7. Papanas N, Ziegler D. Prediabetic neuropathy: Does it exist? Curr Diabetes Rep. 2012;12(4):376-383.
8. Hossain MJ, Al-Mamun M, Islam MR. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci Rep. 2024;7(3):e2004.
9. Hicks CW, Wang D, Matsushita K, et al. Peripheral neuropathy and all-cause and cardiovascular mortality in U.S. adults: A prospective cohort study. Ann Intern Med. 2021;174(2):167-174.
10. Mengstie MA, Chekol Abebe E, Behaile Teklemariam A, et al. Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications. Front Mol Biosci. 2022;9:1002710.
11. Lin Q, Li K, Chen Y, et al. Oxidative stress in diabetic peripheral neuropathy: Pathway and mechanism-based treatment. Mol Neurobiol. 2023;60(8):4574-4594.
12. American Diabetes Association Professional Practice Committee. 12. Retinopathy, neuropathy, and foot care: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(Suppl 1):S185-S194.
13. Bell DSH. Metformin-induced vitamin B12 deficiency can cause or worsen distal symmetrical, autonomic and cardiac neuropathy in the patient with diabetes. Diabetes Obes Metab. 2022;24(8):1423-1428.
14. Sayedali E, Yalin AE, Yalin S. Association between metformin and vitamin B12 deficiency in patients with type 2 diabetes. World J Diabetes. 2023;14(5):585-593.
15. Alvarez M, Sierra OR, Saavedra G, Moreno S. Vitamin B12 deficiency and diabetic neuropathy in patients taking metformin: A cross-sectional study. Endocr Connect. 2019;8(10):1324-1329.
16. Didangelos T, Karlafti E, Kotzakioulafi E, et al. Vitamin B12 supplementation in diabetic neuropathy: A 1-year, randomized, double-blind, placebo-controlled trial. Nutrients. 2021;13(2):395.
17. Bhanja D, Zain A, Moeckel C, Waheed A. Trends in vitamin B12 level testing in patients on metformin from 2000 to 2020. PRiMER. 2024;8:33.
18. Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD007938.
19. Bodman MA, Dreyer MA, Varacallo M. Diabetic peripheral neuropathy. In: StatPearls [Internet]. StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK442009/
20. Armstrong DG, Fisher TK, Lepow B, et al. 26. Pathophysiology and principles of management of the diabetic foot. In: Fitridge R, Thompson M, eds. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]. University of Adelaide Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK534268/
21. Timar B, Timar R, Gaiță L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: A cross-sectional study. PLoS One. 2016;11(4):e0154654.
22. Chan AW, MacFarlane IA, Bowsher D, Campbell JA. Weighted needle pinprick sensory thresholds: A simple test of sensory function in diabetic peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1992;55(1):56-59.
23. Newman G. Merck Manual Professional Division: How to Assess Sensation. Albert Einstein Medical Center; 2023.
24. [No authors listed]. Appendix 11B – Rapid screening for diabetic neuropathy using the 128 Hz vibration tuning fork (the “on-off” method). Can J Diabetes. 2018;42(Suppl 1):S321.
25. Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care. 2001;24(2):250-256.
26. Chatzis DG, Kolokathis K, Magounaki K, et al. Changing the concept: From the traditional glucose-centric to the new cardiorenal-metabolic approach for the treatment of type 2 diabetes. touchREV Endocrinol. 2021;17(2):92-101.
27. Landefeld CS, Steinman MA. The neurontin legacy—marketing through misinformation and manipulation. N Engl J Med. 2009;360(2):103-106.
28. Goodman CW, Brett AS. Gabapentinoids for pain: Potential unintended consequences. Am Fam Physician. 2019;100(11):672-675.
29. U.S. Food and Drug Administration. FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR). Published Jan. 19, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-warns-about-serious-breathing-problems-seizure-and-nerve-pain-medicines-gabapentin-neurontin
30. Premont M, Aungst C. Is gabapentin a controlled substance? In some states, yes. GoodRx. Updated July 26, 2022. https://www.goodrx.com/gabapentin/is-gabapentin-a-controlled-substance
31. Tesfaye S, Boulton AJM, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36(9):2456-2465.
32. Wu CS, Huang YJ, Ko YC, Le CH. Efficacy and safety of duloxetine in painful diabetic peripheral neuropathy: A systematic review and meta-analysis of randomized controlled trials. Syst Rev. 2023;12(1):53.
33. Yang H, Sloan G, Ye Y, et al. New perspective in diabetic neuropathy: From the periphery to the brain, a call for early detection, and precision medicine. Front Endocrinol (Lausanne). 2020;10:929.
34. Kandeel M. The outcomes of sodium-glucose co-transporter 2 inhibitors (SGLT2I) on diabetes-associated neuropathy: A systemic review and meta-analysis. Front Pharmacol. 2022;13:926717.
35. García-Casares N, González-González G, de la Cruz-Cosme C, et al. Effects of GLP-1 receptor agonists on neurological complications of diabetes. Rev Endocr Metab Disord. 2023;24(4):655-672.
36. Nordheim E, Jenssen TG. Chronic kidney disease in patients with diabetes mellitus. Endocr Connect. 2021;10(5):R151-R159.
37. Hussain S, Jamali MC, Habib A, et al. Diabetic kidney disease: An overview of prevalence, risk factors, and biomarkers. Clin Epidemiol Glob Health. 2021;9:2-6.
38. Zemaitis MR, Boll JM, Dreyer MA. Peripheral arterial disease. In: StatPearls [Internet]. StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK430745/
39. Munger MA, Hawkins DW. Atherothrombosis: Epidemiology, pathophysiology, and prevention. J Am Pharm Assoc (2003). 2004;44(2 Suppl 1):S5-12.
40. Ness J, Aronow WS. Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age of 80 years, in an academic hospital-based geriatric practice. J Am Geriatr Soc. 1999;47(10):1255-1256.
41. Molina CS, Faulk JB. Lower extremity amputation. In: StatPearls [Internet]. StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK546594/
42. Geiss LS, Li Y, Hora I, et al. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care. 2019;42(1):50-54.
43. Doucette M. Top ten tips: Realistic expectations about amputation. Wounds International. Published April 24, 2024. https://woundsinternational.com/journal-articles/top-ten-tips-realistic-expectations-about-amputation/
44. Achim A, Stanek A, Homorodean C, et al. Approaches to peripheral artery disease in diabetes: Are there any differences? Int J Environ Res Public Health. 2022;19(16):9801.
45. Bild DE, Selby JV, Sinnock P, et al. Lower extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care. 1989;12(1):24-31.
46. Murabito JM, D’Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication: A risk profile from The Framingham Heart Study. Circulation. 1997;96(1):44-49.
47. Dawson I, van Bockel JH, Brand R. Late nonfatal and fatal cardiac events after infrainguinal bypass for femoropopliteal occlusive disease during a thirty-one-year period. J Vasc Surg. 1993;18(2):249-260.
48. Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review. Circulation. 1996;94(11):3026-3049.
49. McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351(27):2795-2804.
50. Ahmed B, Al-Khaffaf H. Prevalence of significant asymptomatic carotid artery disease in patients with peripheral vascular disease: A meta-analysis. Eur J Vasc Endovasc Surg. 2009;37(3):262-271.
51. Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344(21):1608-1621.
52. Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation. 1995;91(5):1472-1479.
53. Griffin KE, Snyder K, Javid AH, et al. Use of SGLT2i versus DPP-4i as an add-on therapy and the risk of PAD-related surgical events (amputation, stent placement, or vascular surgery): A cohort study in veterans with diabetes. Diabetes Care. 2025;48(3):361-370.
54. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69(11):e71-e126.
55. McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and leg symptoms. JAMA. 2004;292(4):453-461.
56. [No authors listed]. Summaries for patients. Associations between peripheral arterial disease and leg function. Am Intern Med. 2002;136(12):1-32.
57. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463-654.