
An Overview of Audiologic Care for the Primary Care Physician
May 1, 2025
By Jillian Hubertz, AuD; Zachary La Fratta, AuD; Shannon Van Hyfte, AuD; and Melissa Newell, AuD
EXECUTIVE SUMMARY
This article provides a comprehensive guide for primary care providers (PCPs) on the importance of integrating audiologic care into clinical practice. It underscores the public health effect of hearing loss, particularly its associations with cognitive decline, social isolation, and depression. PCPs are encouraged to play proactive roles in screening, identifying, and referring patients for audiologic evaluations and interventions across the lifespan. It also reviews diagnostic tools, prevention strategies, and intervention options, including hearing aids, cochlear implants, and vestibular rehabilitation.
- Early screening and referral: PCPs often are the first to detect hearing or balance concerns and should refer patients who fail screenings or report symptoms such as tinnitus, dizziness, or communication difficulties.
- Screening tools: Tympanometry, otoacoustic emissions, and behavioral pure tone audiometry are valuable, efficient screening tools PCPs can use or interpret for different age groups.
- Vestibular concerns: Delays in motor milestones (e.g., late walking or sitting) or frequent dizziness should prompt referrals for vestibular evaluations, especially in children with hearing loss.
- Intervention options: Beyond hearing aids, patients with conductive or mixed hearing loss or those who do not benefit from hearing aids may be eligible for bone-anchored devices or cochlear implants (60/60 guideline: pure tonal average ≥ 60 dB HL and word recognition score ≤ 60%).
- Hearing loss and cognitive decline: Untreated hearing loss is linked to increased risks of dementia. Early detection and amplification may help preserve cognitive function and quality of life.
- Tinnitus management: PCPs can initiate evaluations for tinnitus and refer for audiologic interventions, such as sound therapy, hearing aids, or counseling support.
- Communication strategies and surveillance: PCPs should educate patients and families on clear communication practices and monitor the ongoing effectiveness of hearing aids or other devices through periodic follow-ups.
Introduction
According to the World Health Organization, approximately 1.5 billion people worldwide live with some degree of hearing loss, with numbers projected to increase significantly in the coming decades as the result of aging populations and environmental factors.1 Hearing healthcare is of paramount importance to primary care providers as research continues to provide evidence for what is known about the importance of hearing and communication from a public health perspective. Hearing has a direct effect on quality of life and has broad implications for cognitive health, mental well-being, and social engagement. Studies have shown that untreated hearing loss is associated with cognitive decline, depression, and social isolation, which can lead to higher healthcare costs.2
Audiologists are licensed professionals with doctoral or clinical doctoral degrees in audiology (AuD) specializing in hearing and balance disorders, diagnosis, and rehabilitation. Audiologists provide a broad range of services, from infancy through older adulthood, including diagnostic assessments of hearing and balance, as well as the fitting and management of hearing aids, cochlear implants, and other assistive technologies.3 Audiologists work collaboratively with a variety of healthcare providers, including physicians (primary care physicians, pediatricians, otolaryngologists, otologists), therapists (speech-language pathologists, occupational therapists, physical therapists), and educators (deaf/hard of hearing educators, general and special educators), and early interventionists.4
Early detection and intervention for all ages leads to better hearing healthcare outcomes and lower costs to healthcare and educational systems. From a developmental standpoint, hearing is included in mandated newborn screening programs because of the effect that language delays have on overall development, including auditory skills, spoken language, and social-emotional and academic outcomes. Early intervention for hearing loss can improve speech and language development in children.5 For adults, early detection and treatment of hearing loss can enhance communication, prevent social isolation, improve cognitive health, reduce the risk of depression, and improve quality of life, and has the potential to improve balance and prevent falls.6-10
The Centers for Disease Control and Prevention (CDC) has emphasized the need for comprehensive hearing healthcare strategies that are accessible and affordable.11 In 2017, the U.S. Food and Drug Administration (FDA) Over-the-Counter (OTC) Hearing Aid Act was passed as part of the FDA Reauthorization Act, with finalized rules for OTC hearing aids published in 2022.12,13 The goal of this act was to improve access to hearing intervention for individuals with mild to moderate hearing loss. With the increasing demand for hearing healthcare services, it is crucial that healthcare systems incorporate audiology as a vital part of primary healthcare to meet the needs of diverse populations.14 Unfortunately, despite evidence supporting the importance of hearing healthcare, a need exists in increasing awareness and hearing health literacy among the general population.15 This article serves as a comprehensive overview of audiology practices, including screening, identification, and intervention for the primary care physician (PCP), since physicians often serve as the primary entry point for patient access to audiologic care.
Prevention
PCPs play a crucial role in the prevention of hearing loss, since they often are the first point of contact for patients and have the ability to influence health behaviors across the lifespan. An estimated 12.5% of children ages 6-19 years and 24% of adults aged 20-69 years have acquired permanent damage to their hearing caused by excessive noise exposure.16,17 Noise-induced hearing loss can occur as the result of repeated exposure to dangerously loud sounds or a single exposure to a very loud sound. Exposure to personal media devices, recreational noise (concerts, sporting events), and occupational noise all can contribute to potential hearing loss. Counseling patients on the use of hearing protection devices, such as earplugs or earmuffs, and safe listening levels (e.g., keeping the volume at or below 60%) can promote hearing conservation. Referring a patient for a baseline audiologic evaluation can provide information on hearing levels prior to exposure to noise.
Ototoxic medication and therapeutics are another consideration for hearing conservation. Physicians should be aware of what treatments pose a risk for hearing loss, such as certain aminoglycoside antibiotics and chemotherapy drugs (e.g., cisplatin and carboplatin) and refer for a baseline audiologic exam and ongoing monitoring if warranted.
The CDC’s website, Stopping Elderly Accidents, Deaths & Injuries (STEADI; https://www.cdc.gov/steadi/index.html), can be a guideline for all medical professionals interested in preventing, screening, assessing, and treating balance disorders while providing detailed education for patients and families. Healthcare providers can recommend referrals for preventive exercises or balance training programs to reduce fall risk in collaboration with physical therapists. Yoga and tai chi are beneficial in rehabilitating vestibular disorders and improving balance.18
Screening
Physicians can categorize hearing screening by age group or test type. Screening tests include those that require no behavioral response, those that require a behavioral response, and questionnaires. PCPs who understand these tests and their implications can explain the results to patients and refer them for diagnostic testing as needed.
Non-Behavioral Hearing Screening Tests
Tympanometry and otoacoustic emissions are two non-behavioral hearing screening tests with distinct purposes. Tympanometry provides information regarding the health and status of the ear canal and middle ear, providing data to quantify visual inspection of the ear. This test uses a probe tip, which requires a seal at the ear canal to calibrate pressure within the outer ear canal space. This test provides information regarding the ear canal size and movement of energy through the eardrum. The patient does not provide a response, and the test generally takes three to five seconds to provide information. Physicians can use tympanometry across the lifespan. A normal tympanogram (Type A) indicates a normal ear canal volume and normal tympanic membrane function, while an abnormal tympanogram can indicate cerumen occlusion (Type B with a small ear canal volume), an open pressure equalization (PE) tube or perforation (Type B with a large ear canal volume), negative middle ear pressure (Type C), or otitis media with effusion (Type B with a normal ear canal volume).19 The American Academy of Pediatrics, American Academy of Family Physicians, and Agency for Healthcare Research and Quality recommend that tympanometry be an optional method to confirm otitis media with effusion.20,21 Physicians should consider referring patients to an otolaryngologist based on case history and abnormal tympanometry results.
Otoacoustic emission screening often is conducted using one particular type of emission called distortion product otoacoustic emissions (DPOAEs). The practitioner places a probe in the patient’s ear canal to obtain a seal. Two sounds are generated and travel through the ear canal and middle ear space to the inner ear/cochlea. When the cochlear outer hair cells detect these signals, they emit their own unique signal that travels back to the middle ear and ear canal, where it is recorded and interpreted. For normal DPOAEs, the ear canal and middle ear must be clear, and the cochlear outer hair cells must be functioning typically. This test applies to all ages but is particularly valuable for pediatric and nonresponsive adult populations. When a patient does not pass the DPOAE, PCPs should refer the patient to an audiologist for a diagnostic assessment.
DPOAEs often are associated with the pediatric population, since they are used in universal newborn hearing screening programs. Newborn hearing screening results are shared with the PCP as the medical home and to encourage support to parents who receive a referral from this test to obtain a diagnostic audiologic evaluation. The Joint Committee on Infant Hearing outlines minimum goals referred to as 1-3-6, in that newborns should be screened by age 1 month, receive diagnosis by age 3 months, and obtain intervention services by age 6 months.23 Many states already meet this goal and then are encouraged to adopt the 1-2-3-month timeline to provide intervention at a younger age. If an infant is not born in a hospital setting, the onus of a hearing screening falls to the PCP, who may refer this process to an audiologist. Additionally, if a child is considered as high-risk for developing hearing loss, a diagnostic hearing evaluation should occur by 9 months of age and regular hearing screening should be completed after that time.23 Risk factors for permanent hearing loss in children and adults are included in Table 1.
Table 1. Risk Factors for Permanent Hearing Loss Prompting Referral to an Audiologist | |
Pediatric23 | Adult36 |
Family history of childhood hearing loss | Family history of hearing loss |
NICU stay > 5 days | Aging (55 years of age or older) |
Hyperbilirubinemia requiring transfusion | History of noise exposure |
Aminoglycoside administration > 5 days | Ototoxic medication (i.e., chemotherapy drugs) |
Asphyxia or hypoxic ischemic encephalopathy | Head trauma |
ECMO | Tumors within the head |
In utero infections (i.e., herpes, rubella, syphilis, toxoplasmosis, CMV) | Autoimmune inner ear disease |
Craniofacial malformations | Cognitive decline/social isolation |
Syndromes associated with hearing loss | Diabetes |
Meningitis | Meningitis |
Caregiver concern | Patient or family concern |
NICU: neonatal intensive care unit; ECMO: extracorporeal membrane oxygenation; CMV: cytomegalovirus | |
DPOAEs also can be useful for older children or adults who are difficult to test or inconsistent with behavioral testing. The test typically takes less than one to two minutes to complete and provides valuable information that may be used in conjunction with tympanometry or as a stand-alone screening test. Generally speaking, most DPOAE equipment provides information on a patient’s higher frequency hearing and, as with all screeners, is viewed as one piece of data along with case history and patient evaluation.24
Behavioral Hearing Screening
In behavioral pure tone audiometry, the practitioner instructs a patient to respond when detecting a soft sound at low, middle, and high frequencies in each ear using an audiometer. The patient faces away from the tester during this process. In screenings, the administrator typically assesses all frequencies (500 Hz, 1,000 Hz, 2,000 Hz, and 4,000 Hz) at a single soft volume (20 dB HL to 25 dB HL). The test takes two to four minutes to complete.
In pediatric screenings, the goal is to identify children likely to have or be at risk of developing permanent hearing loss. Failure to detect congenital or acquired hearing loss in children may result in lifelong deficits in speech and language acquisition, poor academic performance, personal-social maladjustments, and emotional difficulties, according to Harlor et al.25 Children with untreated hearing loss are at greater risk for requiring additional school resources. Although schools conduct periodic pure tone hearing screenings, the screenings do not occur yearly, positioning PCPs as crucial for ongoing hearing monitoring.
PCPs should screen adults who meet age criteria and those experiencing tinnitus or having a history of noise exposure, difficulty understanding speech in noise, or cognitive decline. A 2022 study suggests primary prevention screenings for adults should start at age 30 years for males and age 35 years for females, with secondary prevention beginning at 45 years of age for males and 55 years of age for females.26 The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) provides key action statements in their clinical practice guideline for age-related hearing loss indicating that all patients aged 50 years or older should be screened for hearing loss at their healthcare encounter. If a patient is referred for a hearing screening, providers should consider sociodemographic and patient preferences regarding access and use of hearing healthcare services.27
Case History and Self-Reported Questionnaires
A thorough case history in conjunction with self-reported questionnaires can serve as efficient hearing screening tools in primary care. Patients can complete these screening tools before appointments, allowing physicians to review them together with the patient. Case history interviews can identify risk factors for hearing loss across the lifespan (see Tables 1 and 2). Self-assessments offer a valid and efficient way to gauge an adult patient’s perception of hearing. Studies show that, when pure tone audiometry is unavailable, self-assessment questionnaires accurately determine hearing status.28,29 Based on case history and self-reported questionnaire results, physicians may refer patients to an audiologist for further evaluation.
Table 2. Case History Questions and Self-Assessment Tools for Adults36 | |
Possible Case History Questions | Self-Assessment Tools |
Do you suspect hearing loss in one or both ears? | Hearing Handicap Inventory for the Elderly — Screening Version37 |
Do you hear any ringing or other noises in your head? | Significant Other Assessment of Communication38 |
Do you have a family history of hearing loss? | Self-Assessment of Communication38 |
Have you ever been exposed to loud sounds (occupational or recreational)? | Speech, Spatial, and Qualities of Hearing Scale39 |
Adults wait an average of 8.9 years from the time they first notice difficulty with hearing until the time they take action.30 Hearing loss has been clearly associated with cognitive decline.31,32 Additionally, hearing loss can lead to social isolation, which can contribute to depression.33 PCPs can have a great effect on reducing the time for adults to get a hearing screening and take action related to hearing loss to improve communication function. Earlier intervention in hearing loss is associated with better outcomes, quality of life, and, when used regularly, one study also has found lower risks of mortality.34,35 See Table 2 for optional case history questions and self-assessment tools.
Vestibular Screening
Audiologists screen infants and children with vestibular dysfunction, particularly those at risk because of congenital conditions, ototoxic medications, or genetic factors. (See Table 3.) Vestibular screenings may be integrated into routine audiological assessments for children with hearing loss, particularly those with degrees of hearing loss above 66 dB HL (pure tone average [PTA], the average of hearing levels at 500 Hz, 1,000 Hz, and 2,000 Hz). Motor milestones should be monitored carefully for any suspected delays. In children with hearing loss, walking later than 14 months of age may indicate a vestibular problem. Vestibular dysfunction in children can result in fearful behavior, difficulty moving the head without blurred vision, and difficulty coordinating motor skills.40
Table 3. Four Risk Factors that Should Generate a Vestibular Consult/Evaluation for a Child of Any Age40 | |
Pure tone average greater than 66 dB in one or both ears | Yes/No |
Parental concern for balance | Yes/No |
Walked later than 14 months and has hearing loss | Yes/No |
Sitting later than 7.25 months and has hearing loss | Yes/No |
Screening for balance and dizziness disorders in adults often involves a detailed case history, questionnaires, and functional balance assessments to determine if a more in-depth vestibular assessment is required. Having a patient stand on a firm and foam surface, eyes open and eyes closed, can provide information about the vestibular, proprioceptive, and visual components of balance and when the patient’s balance begins to fail.41
Identification
Comprehensive and tailored audiologic diagnostic testing plays a crucial role in accurately identifying ear- and hearing-related disorders and guiding clinical decision-making for ongoing management. Physicians and clinicians refer patients for a diagnostic audiologic exam when they refer on a screening test or report concerns about hearing, tinnitus, balance, or other ear-related issues. A comprehensive audiologic evaluation typically includes pure tone audiometry, speech audiometry, and additional tests as necessary.
Pure Tone Audiometry
Pure tone audiometry includes air conduction and bone conduction tests, which allow the clinician to determine the degree, configuration, and type of hearing loss. Clinicians measure air conduction using headphones or inserts, which assess the entire auditory pathway, including the outer ear, middle ear, inner ear, and beyond. Bone conduction sends sound directly to the inner ear, bypassing the outer and middle ear. This test helps determine the type of hearing loss. If a difference greater than 10 dB HL exists between air conduction and bone conduction, it indicates a significant air-bone gap (ABG), suggesting that something in the outer or middle ear is impeding the transmission of sound to the inner ear.
Speech Audiometry
Speech audiometry consists of speech reception thresholds (SRT), word recognition scores (WRS), and speech-in-noise testing. SRTs measure the softest level (in dB HL) at which a patient can recognize words. For patients who cannot perform this task, such as those with limited spoken language development, clinicians can use speech detection thresholds (SDT) to determine the softest level at which speech is heard. SRT and SDT offer insight into the reliability of a patient’s responses by comparing them to the PTA (the average hearing levels at 500 Hz, 1,000 Hz, and 2,000 Hz) on the audiogram.
Clinicians perform word recognition testing at a clearly audible level, considering the patient’s hearing thresholds to determine speech understanding abilities in quiet. This test can reveal how ear- or hearing-related issues distort speech perception. Table 4 can help interpret WRS results, showing whether word understanding aligns with or deviates from expected levels based on the degree of sensorineural hearing loss.42
Table 4. Expectation for Maximum WRS Based on Degree of Hearing Level, as Estimated by PTA of 500 Hz, 1,000 Hz, and 2,000 Hz | |
PTA (dB HL) | Maximum WRS |
0 | 100% |
5 | 96% |
10 | 96% |
15 | 92% |
20 | 88% |
25 | 80% |
30 | 76% |
35 | 68% |
40 | 64% |
45 | 56% |
50 | 48% |
55 | 44% |
60 | 36% |
65 | 32% |
70 | 28% |
WRS: word recognition score; PTA: pure tone average Adapted from Dubno JR, Lee FS, Klein AJ, et al. Confidence limits for maximum word-recognition scores. J Speech Lang Hear Res. 1995;38(2):490-502. | |
Speech-in-noise testing is particularly useful for evaluating a patient’s ability to understand speech in realistic environments and can help guide recommendations for amplification and assistive listening devices. It is important to note that speech audiometry word lists are biased toward the English language. While some word lists are available in other languages, the tester must be fluent in that language or use an assistant who can accurately score the patient’s responses. Newer speech audiometry tests have been developed to help monolingual English testers accurately score picture-pointing tasks in Spanish, and a language-independent speech-in-noise test also has been created.43,44
Additional Diagnostic Measures
An audiologist selects additional diagnostic tests beyond the standard hearing evaluation based on the value the test adds to the diagnosis. Tests that will aid in differential diagnosis for the patient are used for an accurate and comprehensive conclusion and recommendations for intervention. Examples of additional diagnostic tests include tympanometry, middle ear muscle reflexes (acoustic reflex testing), otoacoustic emissions (OAEs), auditory evoked potentials (including auditory brainstem response [ABR] testing), and auditory processing evaluations. These tests provide a deeper understanding of an individual’s hearing abilities and clinical presentation.
Immittance Testing
Tympanometry is an objective measure that evaluates the compliance of the middle ear system while air pressure is varied. Clinicians often classify tympanograms using a system developed by James Jerger or by descriptive terms.45 Tympanogram types and interpretations are listed earlier in the Screening section (see Figure 1).
Figure 1. Tympanometry Classification Types |
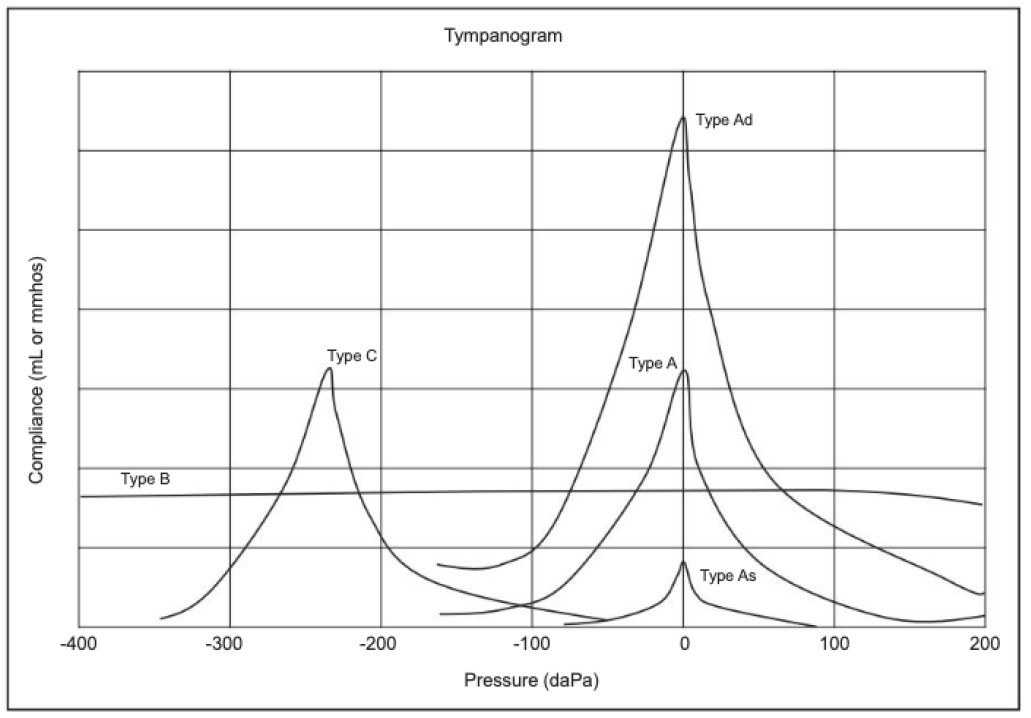 |
From Audiology Review: Preparing for the Praxis and Comprehensive Evaluations (pp. 1-610) by Donai JJ, Fitzharris K. Copyright © 2024 Plural Publishing, Inc. All rights reserved. Used with permission. |
Middle ear muscle reflexes (MEMR) or acoustic reflex threshold testing uses four conditions of measurement to evaluate the outer ear, middle ear, inner ear, and peripheral retrocochlear pathways. The acoustic reflex arc can be found in Figure 1 here: https://www.audiologyonline.com/articles/acoustic-reflex-threshold-art-patterns-875. The pattern of responses from the four conditions measured can provide valuable diagnostic information regarding the site of lesion for auditory pathologies. For a comprehensive tutorial on interpreting acoustic reflex threshold patterns, see “Acoustic Reflex Threshold (ART) Patterns: An Interpretation Guide for Students and Supervisors” by Diane C. Emanuel, PhD, CCC-A (https://www.audiologyonline.com/articles/acoustic-reflex-threshold-art-patterns-875).46
Otoacoustic Emissions
Otoacoustic emissions are generated from the outer hair cells in the cochlea in response to an incoming stimulus and are used as an objective measure to assess cochlear function. DPOAEs and transient evoked otoacoustic emissions (TEOAEs) are used most commonly in clinical practice, primarily with patient populations for whom behavioral audiometry cannot reliably be obtained (e.g., pediatrics, individuals with developmental disabilities). Diagnostically, OAEs also are helpful in identifying changes in cochlear function that might arise prior to being detected via pure tone audiometry, as evidenced in patients undergoing ototoxic monitoring and cases of noise-induced hearing loss.47-49
Auditory Evoked Potentials
Auditory evoked potentials (AEPs), such as the auditory brainstem response (ABR) and auditory steady state response (ASSR), are measured using scalp electrodes and a combination of broad band- and frequency-specific stimuli to estimate hearing sensitivity. AEPs allow clinicians to reliably estimate hearing levels for infants, children, and adults who are not able to complete a behavioral hearing evaluation reliably. They also are used to perform neurodiagnostic evaluations to assess how sound is moving along parts of the peripheral auditory pathway, most often used as a diagnostic tool when peripheral neural function is of concern.
Auditory Processing Evaluation
Auditory processing evaluations are specialized tests that can evaluate central integration of auditory information to measure how auditory input is being processed and understood. A variety of tests exist and should be performed and interpreted by an audiologist who has expertise in this area.
Cross-Check Principle
Synthesizing multiple audiologic tests often leads to the most accurate diagnostic outcome. The cross-check principle is used in audiologic diagnostic testing when the results of one test are levied among results of other tests to determine congruency.50,51 Further investigation is employed when testing results do not align with what is expected to better determine the differential diagnosis. Certain tests, such as tympanometry and otoacoustic emissions, provide information on how one part of the auditory system is functioning, so compiling results to determine the overall result is key to interpretation and accurate diagnosis.
Types of Hearing Loss
Conductive Hearing Loss
There are three types of hearing loss, which are differentiated by the part of the peripheral auditory system that is affected, including conductive hearing loss, sensorineural hearing loss, and mixed hearing loss. (See Figure 2 and Table 5.) Conductive hearing loss occurs when the outer or middle ear is affected, causing sound to not conduct properly to the inner ear, which is typical. On an audiogram, this will present as abnormal air conduction and normal bone conduction, resulting in a significant (> 10 dB HL) ABG. The size of the ABG is influenced by the degree or type of the outer or middle ear pathology. Since the pathology is affecting the conduction of sound, when the clinician is able to amplify speech to an audible level, the patient should perform well with word understanding. Immittance testing, such as tympanometry and middle ear muscle reflexes, will be abnormal in the ear and with conditions that are affected by the outer or middle ear pathology.
Figure 2. Hearing Levels and Hearing Loss |
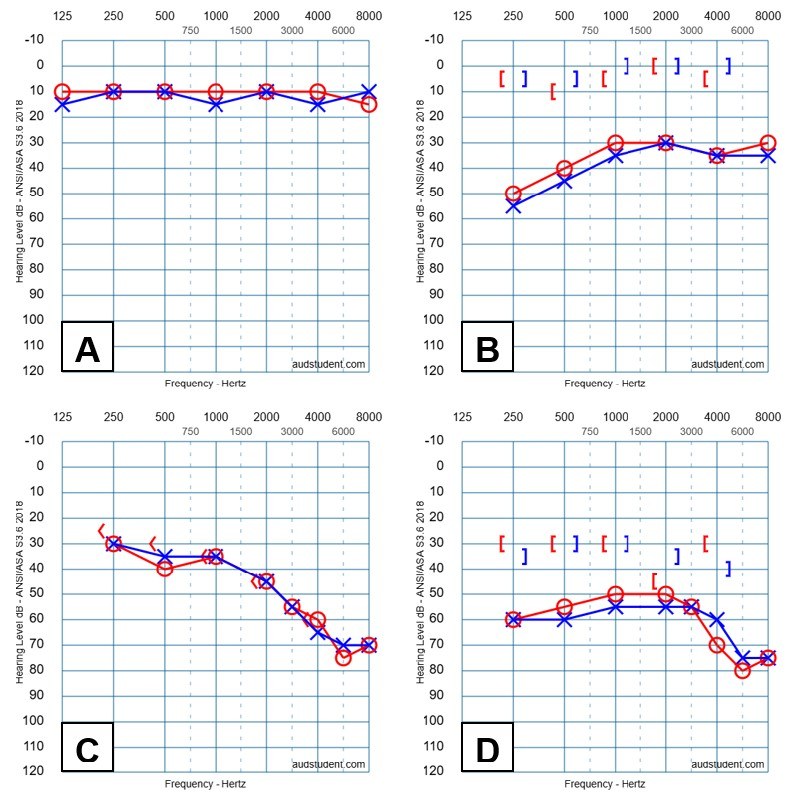 |
A: Normal hearing levels (-10 dB HL to 25 dB HL); B: Conductive hearing loss; C: Sensorineural hearing loss; D: Mixed hearing loss |
Table 5. A Guide to Expected Diagnostic Results Based on the Type of Hearing Loss | ||||
Test | Typical Hearing | Sensorineural Hearing Loss | Conductive Hearing Loss | Mixed Hearing Loss |
Otoscopy |
|
|
|
|
Tympanometry |
|
|
|
|
MEMR (R ipsi, L contra, L ipsi, R contra) |
|
|
|
|
PTA AC |
|
|
|
|
PTA BC |
|
|
|
|
SRT |
|
|
|
|
WRS and speech-in-noise |
|
|
|
|
OAE |
|
|
|
|
WNL: within normal limits; MEMR: middle ear muscle reflex (i.e., acoustic reflex threshold); R: right; L: left; HL: hearing level; CHL: conductive hearing loss; MHL: mixed hearing loss; PTA: pure tone audiometry; AC: air conduction; BC: bone conduction; ABG: air-bone gap; SRT: speech recognition threshold; SNHL: sensorineural hearing loss; WRS: word recognition score; OAE: otoacoustic emission | ||||
Examples of outer or middle ear pathologies that can result in a conductive hearing loss include atresia, cerumen impaction, otitis externa, tympanosclerosis, tympanic membrane perforation, otitis media, cholesteatoma, otosclerosis, ossicular chain disarticulation, and Glomus tumors.52
Sensorineural Hearing Loss
Sensorineural hearing loss (SNHL) occurs when the inner ear, auditory nerve, or central auditory processes are affected. On an audiogram, this will present as abnormal air conduction and abnormal bone conduction, with no significant ABG (< 15 dB HL). Greater degrees of SNHL will result in poorer WRS and speech-in-noise performance, even when sound is at an audible level. Tympanometry typically will be normal and MEMR results will be affected based on the site of the lesion and degree of dysfunction.46 Otoacoustic emissions will be abnormal at frequencies where outer hair cell function is affected. Evoked potentials will be elevated or abnormal. Age-related hearing loss typically occurs in both ears and is symmetric. Asymmetric SNHL occurs where there is a difference in hearing levels between the ears and is cause for a medical referral for evaluation.
No standard currently exists regarding criteria for asymmetry, with several definitions including ≥ 15 dB HL difference at two or more frequencies or ≥ 15% difference in WRS or a > 15 dB HL difference between PTAs of 500 Hz, 1,000 Hz, 2,000 Hz, and 3,000 Hz.53,54 Case history reports of other asymmetric symptoms (i.e., tinnitus, WRS, dizziness) can strengthen the need for a medical workup, which often can include magnetic resonance imaging (MRI) of the internal auditory canals to rule out retrocochlear pathologies, such as a vestibular schwannoma.27
Examples of inner ear pathologies that can result in an SNHL include age-related hearing loss, noise-induced hearing loss, sudden SNHL, genetically linked etiologies, Ménière’s disease, ototoxicity, meningitis, cytomegalovirus (CMV), acoustic neuroma, and auditory neuropathy spectrum disorder.52
Mixed Hearing Loss
Mixed hearing loss is a combination of conductive hearing loss and SNHL, where both outer or middle ear and inner ear, auditory nerve, or central structures are affected. On an audiogram, this will appear at abnormal air conduction and bone conduction, with a significant (> 10 dB HL) ABG. Speech audiometry will be affected by the degree of the sensorineural component of the hearing loss. Tympanometry and MEMR can be abnormal because of the conductive component of the hearing loss. A common hearing disorder that may present as mixed hearing loss with a normal tympanogram is otosclerosis.
Tinnitus Evaluation
For patients who also report tinnitus, often described as a high-pitch ringing or buzzing sound, additional tests are used to further describe the patient’s tinnitus. Pitch matching, loudness matching, and residual inhibition are measures that can further describe the tone and level of the perceived tinnitus and determine if the application of masking noise can alleviate or lessen the perceived tinnitus for a period of time. Self-reported questionnaires, such as the Tinnitus Handicap Inventory (THI) and Tinnitus Functional Index (TFI), can be used to categorize the effect of tinnitus on daily activities and quality of life.55,56 Combined behavioral and self-reported measures can be used to inform the clinical decision when making recommendations for intervention and ongoing monitoring of outcomes.
Cochlear Implant Evaluation
A referral for a cochlear implant evaluation should be provided to patients who currently are wearing hearing aids but find insufficient benefit or have been recommended hearing aids but do not use them because of lack of benefit. When reviewing a patient’s audiogram, the 60/60 guideline can be used to screen individuals who would benefit from a cochlear implant evaluation referral.57 A patient meets the 60/60 guideline when their PTA (average of hearing levels at 500 Hz, 1,000 Hz, and 2,000 Hz) is greater than or equal to 60 dB HL and their WRS is 60% or less. If a patient meets this criterion or has perceived lack of benefit with properly fit amplification, they should be referred to an audiologist who is experienced with providing cochlear implant services for a cochlear implant evaluation.
Vestibular Evaluation
Audiologists use various diagnostic tools to assess the vestibular system’s function. The clinician will triage the patient based on case history information, forming clinical decisions regarding the required diagnostic techniques. Audiologists will work with physicians to obtain appropriate referrals for testing. After testing has been completed, audiologists will interpret the results and make recommendations for follow-up treatment, rehabilitation, and/or medical referrals. Physicians will receive this synthesized report to help direct care deliberately and objectively.
Case History
The evaluation begins with a thorough case history to understand the patient’s symptoms and medical background. Patients are asked to describe their dizziness or vertigo, including onset, duration, triggers, and associated symptoms (e.g., nausea, hearing loss, vision problems).
Past ear infections, head injuries, or surgeries are relevant, as are conditions such as migraines, cardiovascular disease, or neurologic disorders. A medication review is included, since some medications can cause or exacerbate dizziness and imbalance. Understanding how symptoms affect daily activities helps guide the assessment and treatment planning.
Audiologists may use patient-reported outcome measures, such as the Dizziness Handicap Inventory (DHI) or the Vestibular Disorders Activities of Daily Living Scale (VADL), to gauge the effect of vestibular dysfunction on quality of life.58,59 Functional tests, such as the Timed Up and Go (TUG) or the Dynamic Gait Index (DGI), also may be performed to help determine fall risks and the need for professional intervention.60,61 The Hospital Anxiety and Depression Scale (HADS) also can be used to determine if mental health is worsened by dizziness and imbalance disorders.
This detailed approach to vestibular evaluation helps audiologists identify (dys)function of inner ear structures at the peripheral and central levels, ensuring the best outcomes for patients experiencing dizziness and balance disorders. Outcomes of audiological testing can direct more pointed vestibular rehabilitation, improving efficiency and patient outcomes.
Videonystagmography
Videonystagmography (VNG) measures eye movements to evaluate vestibular function. This test battery comprises subtests that measure various portions of the peripheral and central pathways. Induced or spontaneous, nystagmus is measured and correlated with vestibular (dys)function.62
The VNG test battery differentiates between central and peripheral vestibular lesions, evaluates central vestibular compensation, identifies active and acute pathologies, measures the vestibulo-ocular reflex (VOR), and lateralizes peripheral vestibular pathology. A 25% or greater difference is considered a clinically significant asymmetry in VOR function when assessing caloric outcomes.
Oculomotor tests assess how the eyes respond to specific visual and vestibular stimuli. The saccadic, optokinetic, and pursuit systems are measured, assessing central vestibular pathways. The Dix-Hallpike maneuver diagnoses benign paroxysmal positional vertigo (BPPV) by moving the patient’s head into a specific position to see if it triggers vertigo and nystagmus (involuntary eye movements). Positional tests involve moving the patient into various positions (e.g., lying down, turning the head) to see if symptoms occur. Caloric testing uses warm and cool air or water to stimulate each ear’s vestibular system independently, measuring the strength of the vestibular response. An abnormal response can indicate a vestibular weakness on one side. VNG is one of the most common diagnostic tools used to evaluate the vestibular system by recording and analyzing eye movements.
Rotary Chair Testing
Rotary chair testing assesses how the vestibular system responds to rotation. This test measures the VOR by rotating the patient in a chair while recording eye movements. It is beneficial when caloric testing is inconclusive. The test helps evaluate how well the vestibular system compensates for head movements. This assessment helps identify functional VOR reactivity across the lower frequency range, defining the level of central vestibular compensation, detecting central vestibular pathology, and is considered the gold standard for identifying bilateral vestibulopathy.63
Vestibular Evoked Myogenic Potentials
Vestibular evoked myogenic potentials (VEMP) evaluate the function of the otolithic organs (saccule and utricle) and their neural pathways.62 Cervical VEMP (cVEMP) measures responses from the saccule and the vestibulocollic reflex (VCR). This test assesses the function of the inferior branch of the vestibular portion of cranial nerve VIII, the descending motor tract, the VCR, and the saccule. Asymmetries of greater than 35% are considered clinically significant. Ocular VEMP (oVEMP) assesses the utricle and the VOR. This test assesses the function of the superior branch of the vestibular portion of cranial nerve VIII, the descending motor tract, VOR, and/or the utricle. Asymmetries of greater than 34% are considered clinically significant.
Video Head Impulse Test
Video head impulse test (vHIT) assesses the VOR by measuring eye movements in response to quick head movements. vHIT assesses the high-frequency function of the VOR. The patient makes quick head movements while the examiner records the eye response using specialized goggles. If the eyes fail to maintain fixation during these movements, it suggests dysfunction in the semicircular canals. Overt and covert saccades can be measured during the rehabilitation process to determine if central vestibular compensation is improving.62
Computerized Dynamic Posturography
Computerized dynamic posturography (CDP) tests visual, vestibular, and proprioceptive input integration for balance. This test evaluates how well a patient can maintain balance under various conditions by using a force plate to measure shifts in body weight. It helps determine how the visual, vestibular, and proprioceptive systems contribute to postural stability and can guide rehabilitation strategies. Testing can be repeated to measure rehabilitative gains.62
Pediatric Vestibular Assessment
In children, assessments may be adapted to be age-appropriate and engaging. Performing bedside head impulse tests can help determine if corrective saccades are present, indicating vestibular dysfunction. Child-friendly balance tests help identify vestibular deficits that could affect development, motor skills, and overall quality of life. Portions of the test battery may be used depending on the age and skill level of the child. Pediatric vestibular assessments are an emerging area of audiology, with ongoing updates regarding improved assessment techniques and treatment outcomes. Additional tests can be completed as the child ages and is able to participate in testing.64
Monitoring children with vestibular dysfunction for delays in motor milestones, such as sitting, crawling, or walking, is important since early intervention can mitigate long-term developmental effects. Engaging with families and providing them with the necessary resources to support the child’s development and safety is essential.64 Audiologists ensure parents understand the importance of regular follow-ups and care coordination with physicians and therapists.
Medical Evaluation
For patients experiencing ear-, hearing-, or balance-related concerns and/or who require a medical referral for further evaluation, audiologic testing provides valuable information to the healthcare provider who is responsible for their care. Decisions regarding the medical necessity and type of medical intervention and ongoing management often are informed by the results of diagnostic audiologic evaluations.
Medical intervention should include the evaluation and treatment of age-related hearing loss, as well as other conditions, such as asymmetric hearing loss, conductive or mixed hearing loss, or poor word understanding. PCPs should refer to specialists, such as otolaryngologists or otologists, when asymmetric sensorineural hearing loss, conductive hearing loss, mixed hearing loss, or poorer than expected word understanding occurs. Sudden sensorineural hearing loss (SSNHL) is a medical emergency that should be referred to an otolaryngologist or otologist as soon as possible for a comprehensive audiological evaluation and medical intervention. It is ideal for patients to receive medical intervention within 72 hours of the onset of symptoms. PCPs often are the entry point of care, and it is strongly recommended that physicians first distinguish between conductive hearing loss and SSNHL and refer to otology/otolaryngology when signs of SSNHL, such as acute hearing loss, tinnitus, and/or dizziness, are present with a normal outer and middle ear exam.65
Medical evaluation and workup for hearing loss also is warranted for pediatric patients newly identified with hearing loss. PCPs and pediatricians serve as the medical home for the child and coordinate follow-up services, including consultation for medical workup with an otolaryngologist or otologist, ideally one who is familiar with comprehensive pediatric evaluations. Medical workup often includes a comprehensive medical case history and examination by an otolaryngologist by the age of 3 to 6 months, referral to ophthalmology, genetic testing, and imaging studies such as a high-resolution computed tomography (HRCT) or MRI.67 Additional laboratory tests, such as testing for CMV, can help identify etiology and guide early treatment options. An electrocardiogram and urinalysis also are among tests ordered to evaluate if there are other coexisting conditions. If hearing loss is detected early, testing for CMV can inform early treatment decisions.68 The PCP also can aid in establishing enrollment in early intervention (birth to age 3 months) services or school-related services. Parent support services and accompanying therapies, such as speech-language therapy, aural rehabilitation, and/or learning signed languages, also should be supported and monitored by the medical home. Ensuring a child has appropriate access to language and is making appropriate progress is best managed when all providers work collaboratively.
When considering referrals for patients to receive a comprehensive audiologic evaluation, it is important to identify qualified audiologists with the expertise to adequately assess and provide evidence-based recommendations, intervention, and ongoing management. Specifically, when referring a pediatric patient for diagnostic testing, it is important to ensure the audiologist is knowledgeable and familiar with pediatric considerations and modifications that can be used to yield optimal patient and family outcomes. Using tools included in Table 6 can help to identify audiologists’ expertise in a variety of areas.
Table 6. Resources for Finding Audiologists | |
General audiology and specialties, such as pediatrics, tinnitus, balance, implantable devices, auditory processing, etc. | American Speech-Language-Hearing Association ProFind: https://find.asha.org/pro/#sort=relevancy American Academy of Audiology: https://members.audiology.org/cvweb/cgi-bin/memberdll.dll/info?wrp=find-an-audiologist.htm |
Pediatrics | Early Hearing Detection & Intervention Pediatric Audiology Links to Service: |
Balance | The American Institute of Balance: |
Cochlear impants | Cochlear Americas: https://www.cochlear.com/us/en/connect/find-a-clinic Advanced Bionics: https://www.advancedbionics.com/us/en/home/contact-us/2/find-a-clinic MED-EL: |
Tinnitus | American Tinnitus Association: |
As clinicians, it also is important to ensure patient-centered and family-centered care is provided, and centering clinical decisions around the social determinants of health that are particular to a patient and their family.69 By doing so, clinicians can make referrals and recommendations with a holistic view of the patient and proactively navigate barriers that might exist to prevent accessible hearing healthcare.
Intervention
Physicians often encounter patients with auditory and vestibular disorders, including hearing loss, central auditory processing disorder (CAPD), tinnitus, and vestibular dysfunction. In addition to recognizing these conditions in new patients and making initial referrals to audiologists or specialists, physicians also may be required to provide repeat referrals for ongoing or recurrent issues. This includes current hearing aid users with complaints related to device performance, as well as patients seeking ongoing support for managing symptoms of tinnitus or vestibular dysfunction. These conditions can significantly affect communication, quality of life, and overall health. As PCPs, physicians must be able to identify both new cases and recurring issues, ensuring timely referrals and appropriate management. Effective intervention is essential not only to address immediate concerns but also to improve long-term health outcomes. By recognizing these issues, physicians can help implement the appropriate interventions, improving patients’ experiences, enhancing communication, and promoting better social engagement. This section outlines key considerations to guide physicians in managing these disorders effectively.
Amplification
Hearing Aids
Hearing aids are the most common intervention for hearing loss, but there are several other treatment options, including bone-anchored devices and cochlear impants, which are considered for specific types of hearing loss.70 There are three general styles of hearing aids, each offering distinct advantages. Behind-the-ear (BTE) hearing aids are a versatile and reliable option that provide strong amplification and can accommodate a wide range of hearing losses. These devices can be configured with either a custom earpiece (earmold) or a slim tube with either a custom or non-custom earpiece. Receiver-in-the-ear (RITE) and receiver-in-canal (RIC) hearing aids have become increasingly popular because of their discreet design and modern appeal. These styles place the receiver in the ear canal while keeping the microphone and amplifier behind the ear. They are favored by many consumers for their ability to deliver high-quality sound while remaining discreet and comfortable to wear. In-the-ear (ITE) hearing aids are custom-molded to fit the ear, making them ideal for those with dexterity concerns or anatomical restrictions. ITE hearing aids house all components in a single shell that fits directly in the ear, and they are suitable for individuals with mild to severe hearing loss. The choice of hearing aid style depends on the individual’s hearing loss, lifestyle, and personal preferences, ensuring a tailored approach to improving hearing and communication.
Hearing care providers vary, from hearing aid specialists to audiologists, with the latter having more education and clinical training in evaluating and treating hearing loss. Hearing care providers typically are licensed and should be transparent in their clinical practices and decision-making. A comprehensive needs assessment, real ear measurement, and tailored recommendations to include hearing-assistive technology when necessary are key components of a successful hearing aid fitting. Consumers should ensure these metrics are being provided when considering a hearing care provider. Specific details regarding hearing aid technology, the length of the hearing aid trial, pricing, and warranties should be communicated clearly prior to the hearing aid fitting. The selection of style and level of device technology should be made collaboratively among the clinician, patient, and their family (when appropriate), since these can differ to better accommodate hearing and physical needs. Audiologists can explain the benefits and limitations of hearing technology to the patient and what outcomes are realistic to expect.
Implantable Devices
In addition to traditional hearing aids, there are alternative amplification options for individuals who are not candidates for conventional hearing aids. Bone-anchored hearing aids (BAHA) are used for patients with conductive/mixed hearing loss or single-sided deafness (SSD). See Table 7 for candidacy criteria. These devices bypass the outer and middle ear by transmitting sound vibrations directly to the cochlea through bone conduction. BAHAs can be particularly helpful for patients with chronic ear infections or those who cannot use standard hearing aids because of ear canal issues. Cochlear implants are another option for individuals with moderate to profound SNHL or SSD who do not benefit from hearing aids. See Table 8 for candidacy criteria. A CI works by bypassing damaged portions of the ear and directly stimulating the auditory nerve, providing a sense of sound. Although CIs require surgery for implantation, they can offer significant improvements in speech perception and communication for individuals with profound hearing loss.
Table 7. Bone Conduction Hearing Device Candidacy Criteria71-73 | |
Condition | Candidacy Criteria |
Conductive or mixed hearing loss | Bone conduction threshold pure tone average (PTA) ≤ 55 dB, air-bone gap > 30 dB PTA, medical contraindications for conventional hearing aids |
Single-sided deafness (SSD) | Profound sensorineural hearing loss in one ear with normal hearing in the opposite ear |
Table 8. Cochlear Implant Candidacy Criteria74,75 | |
Condition | Candidacy Criteria |
Adults (18 years of age and older) |
|
Children (younger than 18 years of age) |
|
Over-the-Counter
OTC hearing aids are a newer option that may be suitable for patients with mild to moderate hearing loss who require a lower-cost and more accessible solution. OTC hearing aids are available directly to consumers without the need for a prescription, offering a more convenient and affordable alternative to traditional hearing aids. Although OTC devices can improve hearing, they may lack the personalized fitting and advanced features of prescription hearing aids. A study by Swanepoel et al found that, although OTC hearing aids can provide significant benefits in improving hearing, patients receiving these devices may experience suboptimal outcomes in complex listening situations compared to those who receive hearing aids through professional fitting.70 The lack of professional guidance and customized fitting can result in a less tailored solution, potentially affecting overall satisfaction and long-term benefit. Physicians should guide patients in understanding the limitations of OTC devices and, when appropriate, recommend further evaluation by an audiologist for patients with more complex hearing needs, particularly for those with more severe hearing loss or specific audiological requirements.
Each amplification option — whether a traditional hearing aid, bone-anchored device, or cochlear implant — should be selected based on the patient’s specific hearing loss, anatomical considerations, and lifestyle needs, ensuring the most effective approach for improving hearing and communication.
Needs Assessment and Selection
Choosing the appropriate hearing aid style begins with a comprehensive needs assessment to ensure the device best suits the patient’s hearing and lifestyle needs. The key considerations include the type and severity of hearing loss as well as the ability to understand speech in noise, which directly influences the amplification required. Different hearing aid styles offer various fitting ranges and levels of amplification to optimize support based on the patient’s audiometric profile. (See Figures 3 and 4.)
Figure 3. Contextual Needs for Amplification |
 |
ALDs: assistive listening devices |
Figure 4. Key Considerations for Amplification Selection |
 |
HL: hearing level |
Audiometric assessments are critical, especially with tools such as the Speech Intelligibility Index (SII) and speech-in-noise testing. These assessments help determine how well the patient understands speech in real-world environments, particularly in noisy settings, and whether amplification will sufficiently support communication. The SII quantifies speech audibility, ensuring the selection of a hearing aid that aligns with the patient’s needs.
Beyond audiometric testing, medical clearance may be necessary, particularly if the patient has an underlying medical condition affecting the ear or auditory system. Audiologists must verify there are no contraindications, such as infections or anatomical issues, which could affect hearing aid use, especially for patients requiring cochlear implants or BAHAs.
Physicians should be aware of the growing body of evidence linking untreated hearing loss to cognitive decline, including dementia. Dr. Frank Lin’s research, including the ACHIEVE study, shows that individuals with mild hearing loss have twice the risk of dementia, while those with moderate loss have three times the risk.76 Early intervention with hearing aids may help mitigate these risks. Physicians should educate patients about the cognitive benefits of treating hearing loss. However, physicians must caution against overemphasizing cognitive benefits marketed by hearing healthcare providers. Hearing aids should be selected based on a patient’s hearing needs and quality of life, not solely on cognitive outcomes.
Cerumen management is another important consideration. Significant earwax buildup can interfere with hearing aid performance or earmold impressions, so removal may be necessary before fitting the device. Audiologists can provide cerumen management or refer patients to specialists to ensure the ear canal is clear.
Setting realistic expectations is essential. Although hearing aids can enhance hearing, they do not restore normal hearing, particularly in noisy settings where amplification may not fully resolve speech perception challenges. It is important to educate patients about the limitations of their devices and set clear expectations for understanding speech in complex listening environments.
Hardware features, such as wireless connectivity for streaming audio from smartphones or TVs, and telecoils for access to hearing loops, should be considered to enhance the patient’s experience and provide more flexibility in different environments. Battery type also is an important factor, with rechargeable batteries offering convenience and non-rechargeable batteries offering longer life. The patient’s preference for maintenance or performance will guide the decision.
Verification and validation are essential in ensuring that hearing aids meet the patient’s needs. Verification methods such as real ear measurements are used to measure actual amplification levels according to research-based prescriptive targets and confirm that the device delivers appropriate amplification based on the patient’s profile. Validation helps assess whether the hearing aid is addressing the patient’s functional needs. This process includes using patient-reported outcome measures, such as the Hearing Handicap Inventory for the Elderly (HHIE), to document whether the hearing aid is improving the patient’s quality of life.
In addition to these factors, physicians must consider mild or hidden hearing losses, which often are overlooked. Patients with such losses may perform well in quiet environments but struggle in noisy settings. Relying on compensation strategies, such as visual cues or contextual clues, increases cognitive effort and leads to listening fatigue, which negatively affects quality of life.
By considering these factors, healthcare providers can guide patients to the most appropriate hearing aid options for optimal support and comfort. See Table 9 for a summary of best practices in hearing aid selection and fitting.
Table 9. Physician Actions to Support Best Practices in Hearing Aid Selection and Fitting77 |
|
Hearing Assistive Technology
Hearing assistive technology (HAT), also referred to by other common terms such as assistive listening devices (ALD), FM systems, and remote-microphone systems, plays a crucial role in enhancing hearing and improving communication, particularly in specific environments such as classrooms, theaters, or public spaces. Although physicians generally do not directly recommend specific HAT devices, they can recognize when a patient may benefit from them and incorporate this consideration into their practice. Factors to consider include the severity of hearing loss, the need for sound clarity in particular environments, and challenges such as distance, background noise, or the signal-to-noise ratio (SNR). For example, a simple device such as a Pocketalker can be used to amplify sound in one-on-one conversations or smaller groups. Physicians can assess the need for such devices and refer patients to audiologists for more comprehensive evaluation and device recommendations. Additionally, organizations such as the Hearing Loss Association of America (HLAA) or AG Bell, can be valuable resources for patients seeking information on available assistive technology options.
Surveillance and Ongoing Management
Hearing aids require ongoing management to ensure their continued effectiveness. Patients should be scheduled for routine follow-up appointments to assess the performance of their hearing aids, make necessary adjustments to settings, and address any issues. General surveillance includes several follow-up visits after the initial fitting, typically at six-month and/or 12-month intervals, depending on the patient’s needs. During these appointments, physicians should monitor for signs that may indicate the need for a referral back to the managing audiologist. (See Table 10.) Hearing levels should be monitored routinely since hearing devices will need reprogramming if hearing levels change. The use of properly fitting hearing aids does affect the progression of hearing levels. Overamplification potentially can harm a person’s hearing and under-amplification can result in ineffective use, which is why referral to a hearing care provider using best practices is essential.
Table 10. Signs that Patients Need to See Their Dispensing Audiologist | |
Sign | Description |
Improper fit | Hearing aids are uncomfortable, create audible feedback, or cause irritation. |
Inconsistent performance | Amplification is inconsistent or less effective than expected. Patients might describe they are hearing but not understanding speech. |
Worsening tinnitus | Tinnitus symptoms are not managed or have worsened despite hearing aid use. |
Hearing aid discomfort | Issues with sound quality, feedback, or physical discomfort that cannot be resolved through simple adjustments. |
Excessive feedback | Hearing aids produce an undesirable whistling or feedback sound, which also can indicate a need for cerumen management. |
Difficulty in noisy environments | Inability to hear well in noisy environments despite amplification. |
Excessive battery drain | Batteries deplete quickly, indicating possible device malfunction. |
Dissatisfaction/Non-usage | Patient is unhappy with the device or does not wear it regularly because of discomfort or perceived ineffectiveness. |
If the hearing aids are not properly fitted, real ear measurements should be used to verify that the amplification levels are correct. Additionally, if tinnitus symptoms are not adequately managed with the hearing aid, specialized tinnitus management may be required. If the patient experiences difficulties with the hearing aid’s performance or comfort, this could indicate the need for adjustments, repairs, or even a change in hearing aid style. Physicians should be proactive in referring patients back to the audiologist when these issues arise to ensure optimal hearing aid performance.
Patients who are dissatisfied with their hearing aids or who are not wearing them regularly may need additional counseling or adjustments. Factors contributing to dissatisfaction could include discomfort, poor sound quality, excessive feedback, or inadequate amplification in specific environments. Physicians should explore potential barriers to hearing aid use and collaborate with the audiologist to find solutions that enhance the patient’s satisfaction and overall adherence to hearing aid use.
Tinnitus Management
Tinnitus can affect a patient’s quality of life significantly, making its effective management crucial. Identifying triggers, such as existing hearing loss, stress, diet, medications, trauma, and others, is the first step in managing the condition. These triggers can be grouped into anatomical and structural issues (e.g., hearing loss, ear infections, jaw issues, head and neck injuries), physiological and environmental factors (e.g., stress, sleep deprivation, hormonal changes, high blood pressure), and external or lifestyle factors (e.g., noise exposure, diet, medications, sinus pressure).78 Recognizing these triggers is essential for determining appropriate interventions.
Tinnitus can worsen in patients with hearing loss, particularly those with high-frequency SNHL. Progressive hearing loss often correlates with intensifying tinnitus, and patients may experience greater tinnitus intensity in noisy environments. Recognizing these signs (such as worsening tinnitus alongside hearing loss, and its association with high-frequency hearing loss) helps physicians determine if hearing loss is contributing to the condition.78
Counseling plays a crucial role in managing the emotional distress often associated with tinnitus. Cognitive behavioral therapy (CBT) helps manage emotional responses, while tinnitus retraining therapy (TRT) combines sound therapy with counseling. Audiologists provide treatment by assessing hearing status and using amplification devices such as hearing aids or tinnitus maskers to reduce symptoms.
Physicians are key in initiating tinnitus management by identifying triggers, offering reassurance, and making referrals to specialists. Audiologists can assist with hearing evaluations and tinnitus masking, while psychologists offer CBT for emotional support. Referral to an otolaryngologist is recommended if there are medical concerns, such as ear infections or Ménière’s disease.
Central Auditory Processing Disorder
Central auditory processing disorder (CAPD) is diagnosed by audiologists through specialized auditory tests, since it cannot be detected by routine hearing tests. Physicians should be aware that CAPD involves difficulties in processing and interpreting sound in the brain, despite normal hearing thresholds. It is recognized by professional bodies, such as the American Speech-Language-Hearing Association (ASHA) and the American Academy of Audiology (AAA), which provide guidelines for diagnosis and management.79,80 CAPD manifests in various profiles, including temporal processing, decoding difficulties, and challenges with sound localization, speech in noise, or auditory discrimination. Management depends on the specific auditory processing deficits, and treatment may include remote-microphone systems to improve speech clarity in noisy environments, or auditory training therapies to address specific processing deficits. However, audiologists typically do not receive reimbursement for CAPD-related therapies, which may limit access to these services. Physicians are key in identifying signs of CAPD, such as difficulty understanding speech in noisy environments, following directions, or processing complex auditory information. When CAPD is suspected, referring patients to an audiologist for a full evaluation is essential. Physicians also can assist by recommending remote-microphone systems or educational accommodations, such as strategic seating, for children with CAPD. Early identification and intervention are critical for improving outcomes in patients with CAPD, particularly in academic or social settings.
Vestibular Treatment
Audiologists develop or collaborate on individualized vestibular rehabilitation therapy (VRT), often in conjunction with physical therapists, to help patients compensate for or adapt to vestibular deficits. These programs use exercises to improve gaze stability, postural control, and overall balance.81 Education about coping strategies for dizziness, managing lifestyle changes, and understanding the condition is integral to treatment. Audiologists provide ongoing support to help patients manage symptoms effectively and work closely with otolaryngologists, neurologists, physical therapists, and occupational therapists to manage complex cases, especially when patients present with multisystem involvement or require surgical intervention.
Funding
The cost of hearing aids can be a significant barrier for many patients. It is important for physicians to be aware of the various funding options available to ensure access to care. Many health insurance plans, including Medicare supplement plans and Medicaid, may cover part or all of the cost of hearing aids. However, coverage can vary widely depending on the patient’s plan. Patients should be encouraged to check with their insurance provider to verify specific benefits. For patients without insurance coverage, private pay options are available, and payment plans or financing options may help reduce the upfront financial burden.
Additionally, certain states and federal programs, such as vocational rehabilitation services, early intervention, or community programs, may offer funding for hearing aids, especially for pediatric, low-income, or older patients. These programs often have specific eligibility criteria, including age, income level, and hearing loss severity, which patients must meet to qualify for assistance. Physicians should note that audiologists may or may not participate in all funding programs, and it is important for physicians to guide patients to the appropriate resources and referrals for financial assistance.
It also is important to recognize that audiologists do not receive reimbursement for aural rehabilitation (AR) services, which include auditory training and therapy, often an essential part of the hearing aid fitting and adjustment process. This lack of reimbursement can create financial challenges for audiologists and limit access to comprehensive rehabilitation services for patients. Physicians can help advocate for Medicare legislation that would provide coverage for AR services, improving patient access to complete hearing care.
Importance of Communication Strategies
Effective communication strategies are essential for ensuring optimal hearing aid use and improving patient-provider interactions. (See Table 11.) Physicians should engage in clear, open communication with patients about their hearing loss, treatment options, and expectations. This helps set realistic goals for the patient and ensures that the chosen amplification solution fits their needs.
Table 11. Communication Strategies for Patients with Hearing Loss: Guidance for Primary Care Providers82 | |
Communication Strategy | Description |
Face the listener. | Encourage patients to face the person they are communicating with to better read lip movements and facial expressions. |
Use clear speech. | Speak slowly, clearly, and at a normal volume. Avoid shouting or speaking too quickly. |
Reduce background noise. | Advise patients to minimize environmental noise when communicating, such as turning off the TV or moving to a quieter space. |
Provide written or visual cues. | Offer written instructions or use visual aids to reinforce spoken communication. |
Use communication devices. | Encourage use of assistive technologies like hearing aids, FM systems, or other devices for better sound clarity. |
Ask for clarification. | Suggest that patients ask others to repeat themselves or clarify statements when necessary. |
Maintain good lighting. | Make sure there is adequate lighting so the patient can see the speaker’s face and gestures. |
Keep conversations short. | Break long conversations into shorter segments to reduce cognitive load and listening fatigue. |
Use gestures and facial expressions. | Encourage patients to use or observe hand gestures and facial expressions for additional context. |
Ensure one person at a time is speaking. | In group conversations, suggest that only one person speak at a time to reduce confusion and ensure clear understanding. |
In addition, patients may benefit from aural rehabilitation to improve their listening skills and overall communication abilities. This can include auditory training, speech reading (lip reading), and counseling to support the patient’s adaptation to hearing aids. Such approaches also can help reduce listening fatigue and improve the patient’s quality of life.
Summary
In summary, hearing healthcare literacy is important for the PCP from screening to identification to intervention, since PCPs are interfacing with patients across the lifespan. Language development, communication function, and mental well-being are important factors with hearing health and should be considered in patient care assessments. Audiologists hold expertise in screening, identification, and intervention services for hearing loss and associated technologies. Interprofessional collaboration between PCPs and audiologists has immense potential to improve patient outcomes and overall population health.
Jillian Hubertz, AuD, is Assistant Clinical Professor, Department of Speech, Language, Hearing Sciences, Purdue University, West Lafayette, IN.
Zachary La Fratta, AuD, is Assistant Clinical Professor, Department of Speech, Language, Hearing Sciences, Purdue University, West Lafayette, IN.
Shannon Van Hyfte, AuD, is Clinical Professor, Department of Speech, Language, Hearing Sciences, Purdue University, West Lafayette, IN.
Melissa Newell, AuD, is Assistant Clinical Professor, Department of Speech, Language, Hearing Sciences, Purdue University, West Lafayette, IN.
References
1. World Health Organization. World report on hearing. Published March 3, 2021. https://www.who.int/publications/i/item/9789240020481
2. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248): 413-446.
3. American Academy of Audiology. Scope of practice. Updated April 2023. https://www.audiology.org/wp-content/uploads/2023/04/Scope-of-Practice_2023.pdf
4. American Speech-Language-Hearing Association. Interprofessional practice survey results. Published January 2023. https://www.asha.org/siteassets/surveys/2023-interprofessional-practice-survey-results.pdf
5. Chien W, Lin F. Early intervention and its impact on speech development in children with hearing loss. J Speech Lang Hear Res. 2023; 66(4):1207-1219.
6. Liu Y, Kiemle K. The role of early identification and intervention in mitigating the effects of age-related hearing loss. J Clin Audiol. 2020;45(3):183-191.
7. Scarinci N, Worrall L, Hickson L. The effect of hearing impairment in older people on the spouse. Int J Audiol. 2008;47(3):141-151.
8. Chung JH, Bhattacharyya N. Association between hearing loss and cognitive decline in older adults: Early intervention matters. JAMA Otolaryngol Head Neck Surg. 2021;147(2):112-119.
9. Ciorba A, Bianchini C, Pelucchi S, Pastore A. The impact of hearing loss on the quality of life of elderly adults. Clin Interv Aging. 2012;7:159-163.
10. Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med. 2012;172(4):369-371.
11. Centers for Disease Control and Prevention. Hearing loss and the public health cycle. Last reviewed Aug. 10, 2023. https://www.cdc.gov/ncbddd/hearingloss/publichealth.html
12. U.S. Congress. FDA Reauthorization Act of 2017, Public Law No. 115-52. https://www.congress.gov/115/plaws/publ52/PLAW-115publ52.pdf
13. 87 Fed Reg 50698 (Aug. 17, 2022).
14. Liu L, Dong X, Wang J. The need for accessible audiology services in underserved communities. J Public Health Policy. 2021;42(2):234-245.
15. Carlson ML, Nassiri AM, Marinelli JP, et al; Hearing Health Collaborative. Awareness, perceptions, and literacy surrounding hearing loss and hearing rehabilitation among the adult population in the United States. Otol Neurotol. 2022;43(3);e323-e330.
16. Niskar AS, Kieszak SM, Holmes AE, et al. Estimated prevalence of noise-induced hearing threshold shifts among children 6 to 19 years of age: The third National Health and Nutritional Examination Survey, 1988-1994, United States. Pediatrics. 2001;108(1):40-43.
17. Carroll YI, Eichwald J, Scinicariello F, et al. Vital signs: Noise-induced hearing loss among adults — United States 2011-2012. MMWR Morb Mortal Wkly Rep. 2017;66(5):139-144.
18. Cronin G. Tai chi for balance. Vestibular Disorders Association. https://vestibular.org/article/diagnosis-treatment/treatments/complementary-alternative-medicine/tai-chi-for-balance/
19. Onusko E. Tympanometry. Am Fam Physician. 2004;70(9):1713-1720.
20. American Academy of Family Physicians; American Academy of Otolaryngology-Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Otitis media with effusion. Pediatrics. 2004;113(5):1412-1429.
21. [No authors listed]. Managing otitis media with effusion in young children. American Academy of Pediatrics The Otitis Media Guideline Panel. Pediatrics. 1994;94(5):766-773.
22. Donai JJ, Fitzharris K. Audiology Review: Preparing for the Praxis and Comprehensive Evaluations. Plural Publishing; 2024.
23. Joint Committee on Infant Hearing. Year 2019 position statement: Principles and guidelines for early hearing detection and intervention programs. J Early Hear Detect Interv. 2019;4(2):1-44. https://www.infanthearing.org/nhstc/docs/Year%202019%20JCIH%20Position%20Statement.pdf
24. Blankenship CM, Hunter LL, Keefe DH, et al. Optimizing clinical interpretation of distortion product otoacoustic emissions in infants. Ear Hear. 2018;39(6):1075-1090.
25. Harlor ADB Jr, Bower C; Committee on Practice and Ambulatory Medicine; Section on Otolaryngology-Head and Neck Surgery. Hearing assessment in infants and children: Recommendations beyond neonatal screening. Pediatrics. 2009;124(4):1252-1263.
26. Jordan J, Baiduc RR, Spankovich C. Hearing screening age considerations for adults: National Health and Nutrition Examination Survey. J Am Acad Audiol. 2022;33(2):58-65.
27. Tsai Do BS, Bush ML, Weinreich HM, et al. Clinical Practice Guideline: Age-related hearing loss. Otolaryngol Head Neck Surg. 2024;170(Suppl2):S1-S54.
28. Ferrite S, Santana VS, Marshall SW. Validity of self-reported hearing loss in adults: Performance of three single questions. Rev Saude Publica. 2011;45(5):824-830.
29. Sindhusake D, Mitchell P, Smith W, et al. Validation of self-reported hearing loss. The Blue Mountains Hearing Study. Int J Epidemiol. 2001;30(6):1371-1378.
30. Simpson AN, Matthews LJ, Cassarly C, Dubno JR. Time from hearing-aid candidacy to hearing aid adoption: A longitudinal cohort study. Ear Hear. 2019;40(3):468-476.
31. Lin FR, Metter EJ, O Brien RJ, et al. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214-220.
32. Liu CM, Lee CTC. Association of hearing loss with dementia. JAMA Netw Open. 2019;2(7):e198112.
33. World Health Organization. Deafness and hearing loss. Published Feb. 26, 2025. https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss
34. Zahl SM. Effects of receiving hearing aids on health-related quality of life in adults with mild hearing loss. J Audiol Otol. 2023;27(1):24-29.
35. Choi JS, Adams ME, Crimmins EM, et al. Association between hearing aid use and mortality in adults with hearing loss in the USA: A mortality follow-up study of a cross-sectional cohort. Lancet Healthy Longev. 2024;5(1):e66-e75.
36. American Speech-Language-Hearing Association. Hearing loss in adults. https://www.asha.org/Practice-Portal/Clinical-Topics/Hearing-Loss/
37. Lichtenstein MJ, Bess FH, Logan SA. Validation of screening tools for identifying hearing-impaired elderly in primary care. JAMA. 1988;259(19):2875-2878. [Erratum in: JAMA. 1990;264(1):38].
38. Schow RL, Nerbonne MA. Communication screening profile: Use with elderly clients. Ear Hear. 1982;3(3):135-147.
39. Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int J Audiol. 2004;43(2):85-99.
40. Janky KL, Thomas MLA, High RR, et al. Predictive factors for vestibular loss in children with hearing loss. Am J Audiol. 2018;27(1):137-146.
41. Cohen H, Blatchly CA, Gombash LL. A study of the clinical test of sensory interaction and balance. Phys Ther. 1993;73(6):346-351; discussion 351-354.
42. Dubno JR, Lee FS, Klein AJ, et al. Confidence limits for maximum word-recognition scores. J Speech Lang Hear Res. 1995;38(2):490-502.
43. Mendel LL, Pousson MA, Bass JK, et al. Spanish Pediatric Picture Identification Test. Am J Audiol. 2020;29(3):318-328.
44. Zaar J, Simonsen LB, Sanchez-Lopez R, Laugesen S. The Audible Contrast Threshold (ACT) test: A clinical spectro-temporal modulation detection test. Hear Res. 2024;453:109103.
45. Jerger J. Clinical experience with impedance audiometry. Arch Otolaryngol. 1970;92(4):311-324.
46. Emanuel DC. Acoustic reflex threshold (ART) patterns: An interpretation guide for students and supervisors. Audiology Online. Published Sept. 14, 2009. https://www.audiologyonline.com/articles/acoustic-reflex-threshold-art-patterns-875
47. Knight KR, Kraemer DF, Winter C, Neuwelt EA. Early changes in auditory function as a result of platinum chemotherapy: Use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25(10):1190-1195.
48. da Silva Barros SM, Frota S, Tavares Atherino CC, Osterne F. The efficiency of otoacoustic emissions and pure-tone audiometry in the detection of temporary auditory changes after exposure to high sound pressure levels. Braz J Otorhinolaryngol. 2007;73(5):592-598.
49. Hotz MA, Probst R, Harris FP, Hauser R. Monitoring the effects of noise exposure using transiently evoked otoacoustic emissions. Acta Otolaryngol. 1993;113(4):478-482.
50. Jerger JF, Hayes D. The cross-check principle in pediatric audiometry. Arch Otolaryngol. 1976;102(10):614-620.
51. Hall JW 3rd. Crosscheck principle in pediatric audiology today: A 40-year perspective. J Audiol Otol. 2016;20(2):59-67.
52. Kramer S, Brown DK. Audiology: Science to Practice. Third edition. Plural Publishing; 2019.
53. Cueva RA. Auditory brainstem response versus magnetic resonance imaging for the evaluation of asymmetric sensorineural hearing loss. Laryngoscope. 2004;114(10):1686-1692.
54. American Academy of Otolaryngology-Head and Neck Surgery. Position statement: Red flags — warning of ear disease. Published April 21, 2021. https://www.entnet.org/resource/position-statement-red-flags-warning-of-ear-disease/
55. Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143-148.
56. Meikle MB, Henry JA, Griest SE, et al. The tinnitus functional index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012;33(2):153-176.
57. Zwolan TA, Schvartz-Leyzac KC, Pleasant T. Development of a 60/60 guideline for referring adults for a traditional cochlear implant candidacy evaluation. Otol Neurotol. 2020;41(7):895-900.
58. Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116(4):424-427.
59. Cohen HS. Use of the Vestibular Disorders Activities of Daily Living Scale to describe functional limitations in patients with vestibular disorders. J Vestib Res. 2014;24(1):33-38.
60. Kear BM, Guck TP, McGaha AL. Timed Up and Go (TUG) test: Normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13.
61. Yang Y, Wang Y, Zhou Y, et al. Reliability of functional gait assessment in patients with Parkinson disease: Interrater and interrater reliability and internal consistency. Medicine (Baltimore). 2016;95(34):e4545.
62. Jacobson GP, Shepard NT, Barin K, et al. Balance Function Assessment and Management. Third edition. Plural Publishing; 2020.
63. Zalewski CK. Rotational Vestibular Assessment. Plural Publishing; 2018.
64. O’Reilly RC, Morlet T, Brodsky JR, Cushing SL. Manual of Pediatric Balance Disorders. Second Edition. Plural Publishing; 2020.
65. Chandrasekhar SS, Tsai Do BS, Schwartz SR, et al. Clinical Practice Guideline: Sudden hearing loss (update). Otolaryngol Head Neck Surg. 2019;161(1_suppl):S1-S45.
66. 81 Fed Reg 89469 (Dec. 12, 2016).
67. Kılıç S, Bouzaher MH, Cohen MS, et al. Comprehensive medical evaluation of pediatric bilateral sensorineural hearing loss. Laryngoscope Investig Otolaryngol. 2021;6(5):1196-1207.
68. Singh G, Gaidhane A. A review of sensorineural hearing loss in congenital cytomegalovirus infection. Cureus. 2022;14(10):e30703.
69. Healthy People 2030. Social determinants of health. U.S. Department of Health and Human Services. https://odphp.health.gov/healthypeople/priority-areas/social-determinants-health
70. Swanepoel DW, Oosthuizen I, Graham MA, Manchaiah V. Comparing hearing aid outcomes in adults using over-the-counter and hearing care professional service delivery models. Am J Audiol. 2023;32(2):314-322.
71. Cochlear. Candidacy, evaluation and fitting protocol. https://assets.cochlear.com/api/public/content/BUN975-BC-Candidacy-Evaluation-and-Fitting-Protocol.pdf
72. MED-EL. Indications. MED-EL. Indications. https://www.medel.pro/indications
73. Oticon Medical. Bone conduction hearing. https://www.oticonmedical.com/
74. Cochlear. Cochlear implant candidacy information. https://www.cochlear.com/us/en/professionals/products-and-candidacy/candidacy/cochlear-implant
75. American Cochlear Implant Alliance. Determining CI candidacy. https://www.acialliance.org/page/determiningcicandidacy
76. Lin F. Hearing loss and cognitive decline: Results of the ACHIEVE study. Bulletin: American Academy of Otalaryngology-Head and Neck Surgery. Published Oct. 1, 2023. https://bulletin.entnet.org/clinical-patient-care/article/22873554/hearing-loss-and-cognitive-decline-results-of-the-achieve-study
77. American Speech-Language-Hearing Association. Preferred practice patterns for the profession of audiology. https://www.asha.org/policy/pp2006-00274/
78. Langguth B, de Ridder D, Schlee W, Kleinjung T. Tinnitus: Clinical insights in its pathophysiology a perspective. J Assoc Res Otolaryngol. 2024;25(3):249-258.
79. American Speech-Language-Hearing Association. Central auditory processing disorders: The role of the audiologist. https://www.asha.org/policy/PS2005-00114/
80. American Academy of Audiology. Clinical Practice Guidelines: Diagnosis, Treatment, and Management of Children and Adults with Central Auditory Processing Disorder. Published Aug. 10, 2010. https://www.audiology.org/practice-guideline/clinical-practice-guidelines-diagnosis-treatment-and-management-of-children-and-adults-with-central-auditory-processing-disorder/
81. Jacobson GP, Shepard NT, Barin K, et al. Balance Function Assessment and Management. Third edition. Plural Publishing; 2021.
82. American Speech-Language-Hearing Association. Tips for communicating with someone who has hearing loss. https://www.asha.org/about/press-room/articles/tips-for-communicating-with-someone-who-has-hearing-loss/?srsltid=AfmBOop4oxMx3pD9oNIud3f49JdU2TMwn7Ko_v_IZAvNufn7emBA3tGu